Over the years, researchers have tried hard to comprehend topographic signals that promote cell mechanical sensitive responses. The extracellular matrix (ECM) provides a complex cellular microenvironment that controls cellular behavior. Nevertheless, only a few functions of these factors are understood, and most remain obscure.
An article published in Advanced Sciences presented a convenient method to demonstrate the curved structure of the ECM network that regulates stem cell mechanotransduction. Here, an ECM-mimicking nanofiber network was prepared using electrospinning technology.

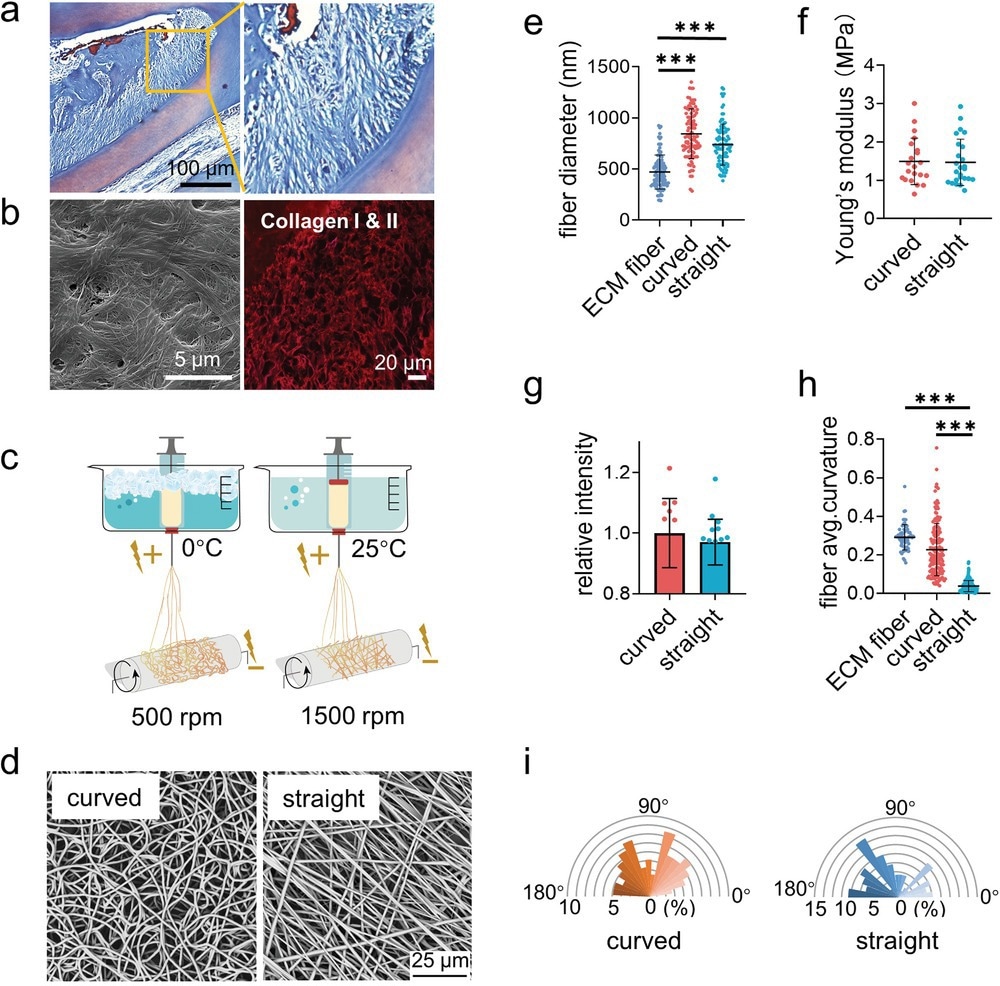
Figure 1. Fabrication and characterization of the curved and straight nanofiber network. a) The Representative images of masson staining of the periodontal tissues. b) The SEM (left) image of the decellularized periodontal ligament tissues and the representative fluorescence image of the collagen I and II (right) in periodontal tissues. c) Scheme of the curved and straight nanofiber network fabrication. The curved and straight fiber network require 0 °C and 25 °C electrospinning temperature, respectively. d) The representative SEM images of the curved and straight fibers (three technical replicates). e) The diameter of the ECM fibers in the periodontal tissues and the artificial fibers (n = 100, two technical replicates). f) Young’s modulus of the curved and straight nanofiber network as detected by Nanoindenter (n = 20, two technical replicates). g) Specific surface area of the curved and straight surfaces as detached by the fluorescent intensity of the adsorbed FITC-BSA at 562 nm (n = 12, two technical replicates). h) The average curvature of the ECM fibers in the periodontal tissues and the artificial fibers (n = 160, two technical replicates). i) The orientation angles (n = 100, two technical replicates) of the curved and straight fibers.
The curved nanofiber promoted cell bridge formation due to cytoskeleton tension. Moreover, the myosin-II-based intracellular force generated by the actomyosin filaments inclined the cell lineage towards osteogenic differentiation. Thus, the present study has provided a better understanding of the effects of topographic signals on cell behavior, thereby aiding the development of new biomaterials.
Effect of Nanofibers on the Functioning of Stem Cells
According to recent studies, the physiological and behavioral functions of cells are influenced by biochemical and physical factors. Novel biomaterials that mimic ECM’s stiffness, degradation, ligand diffusion, stress relaxation, and other physical properties, in addition to the usual chemical effects, have been created.
Nanomaterials, such as nanofibers, are mostly fabricated through electrospinning. In this process, a strong electric field is used to transform solution-based polymers into continuous nanometer-sized fibers.
Various nanofibers differ in their properties, including surface-to-volume ratio and morphology. These characteristics can be altered based on the polymer and intended application. The electrospinning parameters, solution parameters, and ambient characteristics affect the properties of the nanofibers.
Stem cells can develop into various cell types and construct any tissue in the body. However, stem cells have low vitality and are challenging to multiply, which limits their application for a wider range of prospective therapeutic benefits.
Stem cells and electrospun nanofibers have two key advantages. First, by changing the chemical characteristics of the nanofibers to enhance their interactions with stem cells, they can operate as advantageous scaffolds for maintaining stem cells. Second, stem cells can be delivered using nanofibers to particular tissues or organs for tissue engineering and wound repair.
Previous reports have suggested that cancer cells unbend the curled collagen fibers in the ECM during tumor growth. Although curved structures in the fibrous connective tissue, known as the periodontal ligament, were previously known, their function at the cellular level remains unclear. Moreover, studies in this area have been restricted by the absence of techniques for creating curved nanofibers.
Curved Nanofibers to Promote Stem Cell Mechanotransduction
Despite previous reports on electrospinning technology to fabricate biomaterials that mimic the ECM, only a few reports have described the fabrication of curved nanofibers. On the other hand, other studies that carried out low-temperature electrospinning have focused on the porosity of the matrix rather than the topology of nanofibers.
In this study, cryogenic electrospinning technology was utilized to fabricate ECM-mimicking curved nanofibers as a tool to study cell response when exposed to curved structures. Interestingly, curved nanofibers influenced the behavior of stem cells, altering their adhesive nature compared to straight nanofibers.
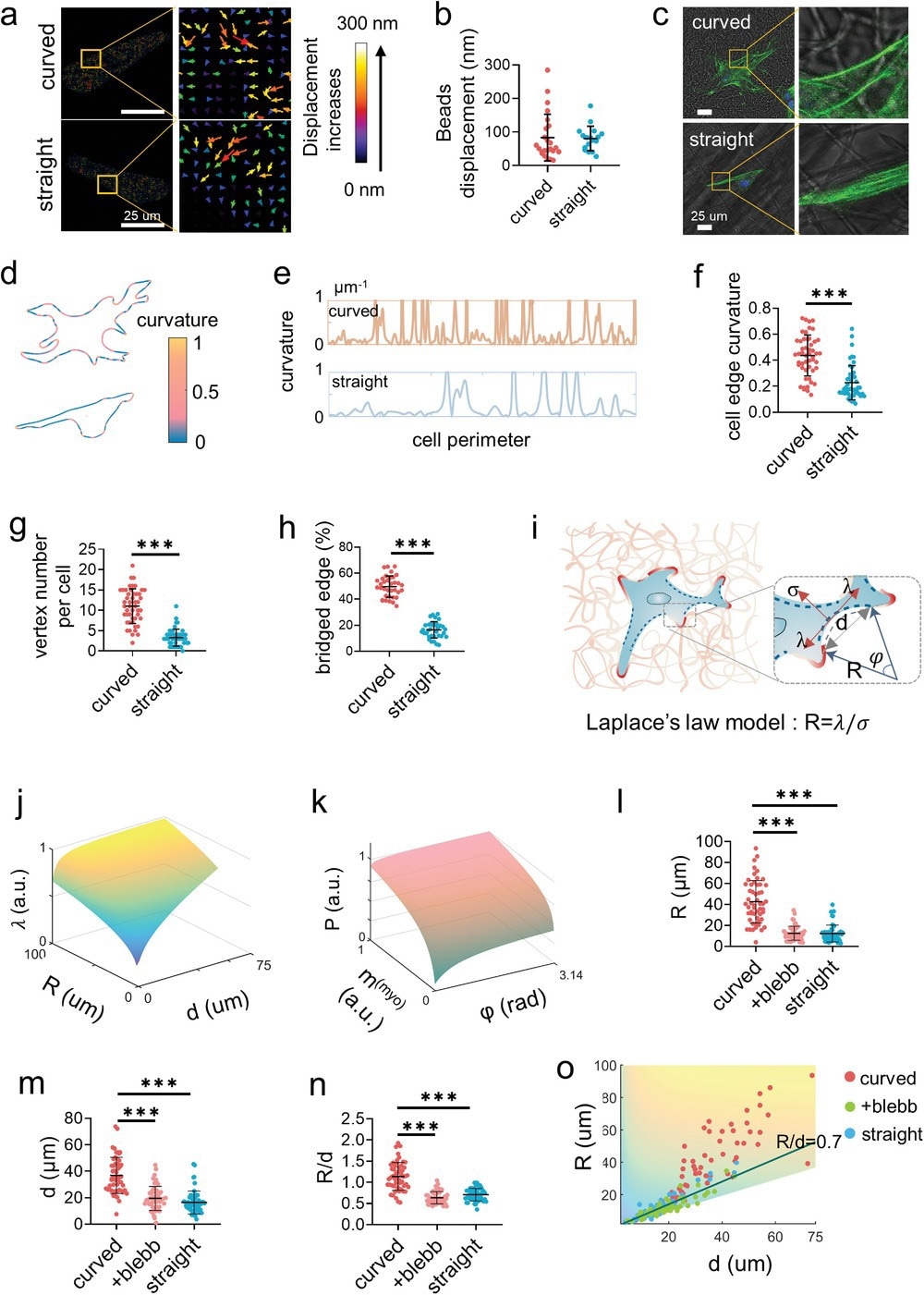
While cells adhered along straight nanofibers, they crossed curved nanofibers to form cell bridges, indicating that the cell bodies overhung instead of attaching to the nanofibers.
The formation of cell bridges rearranged the distribution of the actomyosin cytoskeleton and imparted extra intracellular force, enhancing stem cell mechanotransduction and promoting osteogenic differentiation. The new findings of this study helped obtain a better understanding of the crucial role of biomechanical principles in promoting the development of tissue engineering.
Thus, the present investigation of cell mechanosensing revealed that, while the cell boundary was frequently parallel to the surrounding straight nanofibers, it invariably traversed multiple curved nanofibers as bridges. The cells on the curved nanofibers had a significant percentage of unbound borders that formed large radial arcs that bowed inwards.
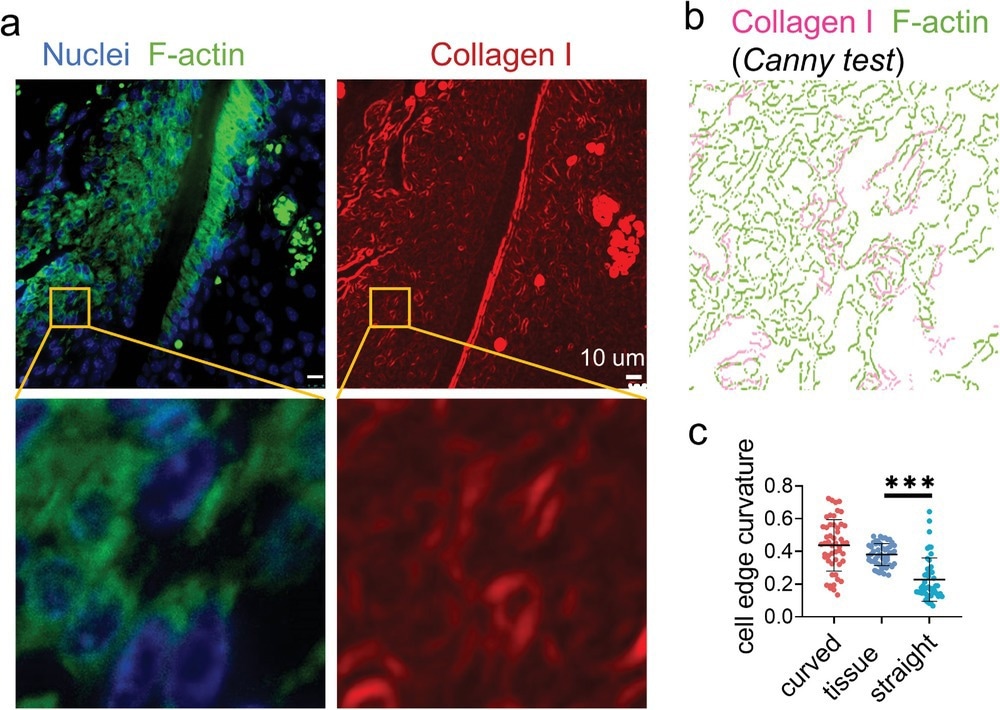
Figure 3. Immunofluorescence staining displays widely distributed cell bridges in the periodontal ligament. a) The representative fluorescence images of nuclei (blue), F-actin (green), and collagen I (red) staining of the mouse periodontal ligament. b) Canny edge test image of the yellow box area in (a). The magenta and green represent the collagen I and F-actin, respectively. c) The average curvature of the cell edges (n = 50, two technical replicates) of the cells in periodontal ligament and cultured on the artificial fibers.
Conclusion
In summary, a simple electrospinning technology that operates at a low speed and temperature to fabricate ECM-mimicking curved nanofiber structures was developed. While the curved nanofibers promoted discrete adhesion in stem cells, straight networks induced the formation of continuous adhesion by stem cells along with the fiber structure.
The curved nanofibers stimulated stem cell mechanotransduction by forming a cell bridge, thereby promoting osteogenic differentiation and proliferation of stem cells. Inducing mechanotransduction and mechanosensing signaling pathways via the formation of nonadhesive bridges caused actomyosin to aggregate and contract.
Thus, the present study demonstrated that the knowledge of cell mechanosensing and tissue development could be improved by using this curved matrix to enhance the database of biomaterials that mimic the ECM.
News
Specially engineered antibody delivers RNA therapy to treatment-resistant tumors
Elias Quijano, PhD; Diana Martinez-Saucedo, PhD; Zaira Ianniello, PhD; and Natasha Pinto-Medici, PhD, there are 25 other contributors, most from Yale's Department of Therapeutic Radiology and from the departments of genetics, molecular biophysics and [...]
Vaccinated women face fewer cervical cancer risks
New data from Denmark shows the HPV vaccine’s powerful long-term impact, while also revealing why cervical cancer screening is still essential. A Danish study published in the journal Eurosurveillance reports that women who received the human [...]
3D-printed implant offers a potential new route to repair spinal cord injuries
A research team at RCSI University of Medicine and Health Sciences has developed a 3-D printed implant to deliver electrical stimulation to injured areas of the spinal cord, offering a potential new route to [...]
Nanocrystals Carrying Radioisotopes Offer New Hope for Cancer Treatment
The Science Scientists have developed tiny nanocrystal particles made up of isotopes of the elements lanthanum, vanadium, and oxygen for use in treating cancer. These crystals are smaller than many microbes and can carry isotopes of [...]
New Once-a-Week Shot Promises Life-Changing Relief for Parkinson’s Patients
A once-a-week shot from Australian scientists could spare people with Parkinson’s the grind of taking pills several times a day. The tiny, biodegradable gel sits under the skin and releases steady doses of two [...]
Weekly injectable drug offers hope for Parkinson’s patients
A new weekly injectable drug could transform the lives of more than eight million people living with Parkinson's disease, potentially replacing the need for multiple daily tablets. Scientists from the University of South Australia [...]
Most Plastic in the Ocean Is Invisible—And Deadly
Nanoplastics—particles smaller than a human hair—can pass through cell walls and enter the food web. New research suggest 27 million metric tons of nanoplastics are spread across just the top layer of the North [...]
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]