NanoApps Medical Near-Term Projects
NanoApps Medical is aiming to investigate the possibility that superparamagnetic nanoparticles (SPIONs) (Figure 1) and other classes of nanoparticles (e.g., gold coated nanoshells) (Figure 2) might have the capacity to target cancerous tumors, metastasizing cancer cells, pathogens, etc. to deactivate/eliminate them via hypothermia. This means that once the nanoparticles are adhered to their targets, they would be heated by an external source (a magnetic field in the case of SPIONs, and near-infrared laser light in the case of gold nanoshells) to inflict irreversible thermal damage to these entities through the catastrophic disruption of their cell membranes. Additionally, gold nanoshells may be synthesized to be hollow, which means that they might be loaded with powerful drugs to impart a dual activity against their targets. A further advantage of this strategy is that the “collateral damage” to surrounding healthy cells will be minimized as the thermal “blast zones” and drug delivery would be highly localized, in stark contrast to conventional chemotherapies that flood the patient with toxic chemicals.
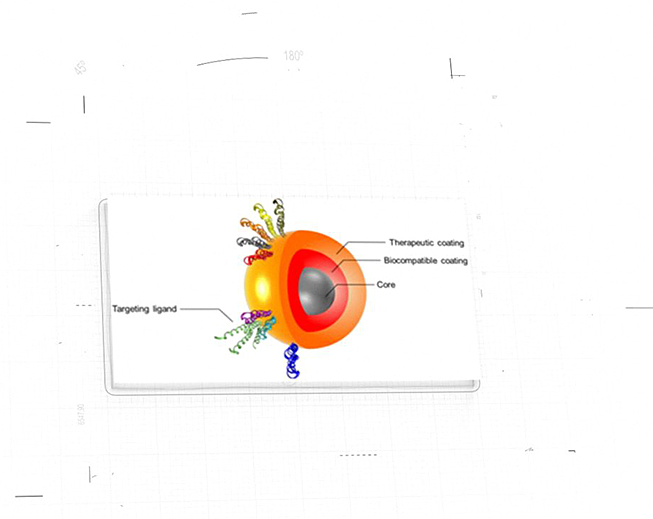
Figure 1: Graphic of “decorated” superparamagnetic nanoparticle (SPION) (Image Credit: Gobbo et al.)
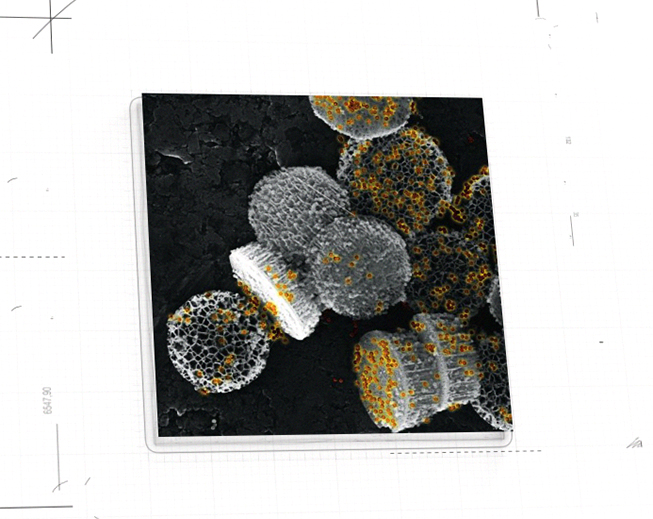
Figure 2: Nanostructures comprised of hollow gold nanoshells nested within silicon discs. (Image credit: John Uhlrich)
NanoApps Medical is also currently seeking to secure funding toward the development of a hybrid DNA/nanoparticle based nanomedical platform (DuFlexx) (Figure 3) for targeted dual action diagnostic and therapeutic applications to address myriad conditions. One of these applications would involve the precise delivery of bone rebuilding materials to fill the voids of bones that have been damaged via osteoporosis. In space applications, the Duflexx platform might be employed to “top dress” the bones of astronauts to negate the effects of bone loss in the space environment, particularly for extended voyages to Mars and beyond.
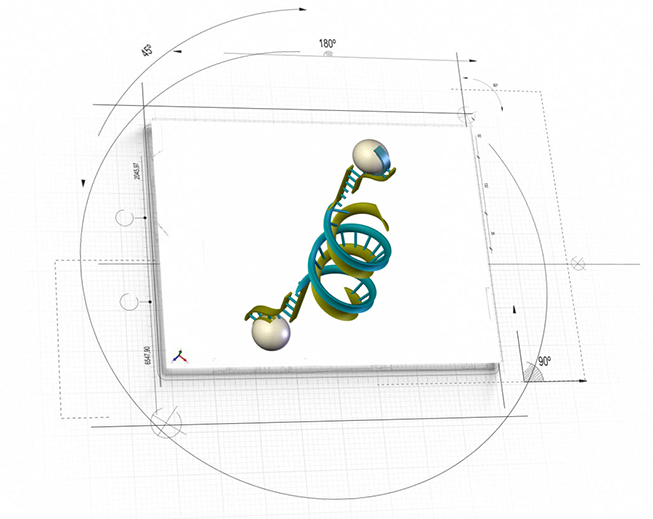
Figure 3: Artistic representation of hybrid DNA/nanoparticle nanomedical platform (DuFlexx) (Image Credit: NanoApps Medical, Inc.)
Toward addressing the still devastating global impact of malaria, NanoApps Medical aims to develop a nanobiosensor (Figure 4) for the detection of malaria via saliva, which would be far less invasive while conveying more rapid results than what is possible with blood samples, which are currently used (blood smears) as the diagnostic standard.
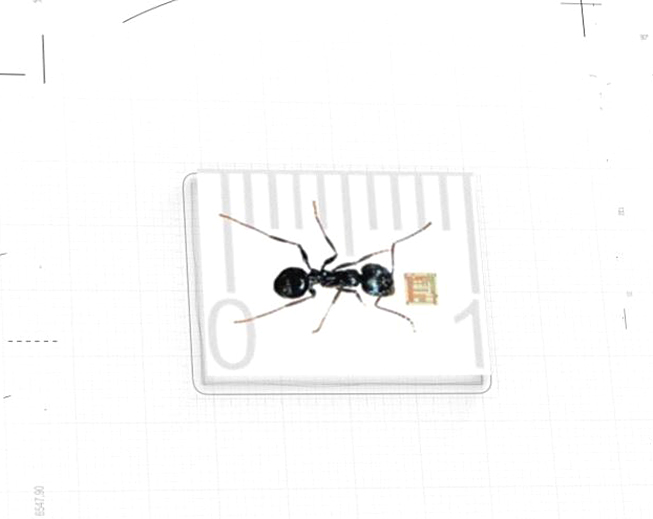
Figure 4: Representation of the scale of a bionanosensor chip (Image credit: Fernanadez)
On the therapeutic side, NanoApps is aiming to develop a nanomedical strategy against malaria, which would involve the disablement of Plasmodium falciparum sporozoites (Figure 5) which are injected into the skin along with the saliva of the Anopheles mosquito. There is an approximately ten minute window between P. falciparum sporozoite injection, their burrowing through the dermal layers to the bloodstream, and several transits in the circulatory system, before they invade the hepatic cells of the liver. The premise is that if the sporozoites can be disabled via nanomedical means, prior to their invasion of the liver, the infection will not be able to proceed.
Figure 5: Plasmodium falciparum sporozoite (Image credit: Microbiology 2009)