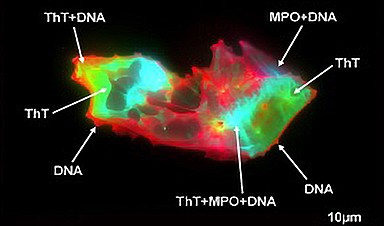
Researchers found persistent microclot and NET structures in Long COVID blood that may explain long-lasting symptoms.
Researchers examining Long COVID have identified a structural connection between circulating microclots and neutrophil extracellular traps (NETs). The discovery indicates that the two may interact in the body in ways that could lead to harmful effects when these processes become unregulated.
Understanding Microclots
Microclots are abnormal clusters of blood clotting proteins that move through the bloodstream. The term entered scientific use in 2021, when Prof Resia Pretorius from Stellenbosch University's Department of Physiological Sciences reported finding these unusual microclots in blood samples from people with COVID-19. The observation received widespread attention because of its potential relevance to clotting complications seen during the pandemic.
What NETs Do in the Immune System
Dr. Alain Thierry and his team at the Montpellier Cancer Institute (IRCM) at INSERM in Montpellier were among the first to show that NETs play a major role in COVID-19. NETs form during a process called NETosis, in which neutrophils release their DNA to create thin strands coated with enzymes that quickly trap and neutralize harmful microbes.
Although NETs are part of the body's defenses, producing them in excessive amounts can contribute to inflammatory and clotting disorders. Such overproduction has been associated with severe infections, autoimmune diseases, cancer, diabetes, and arthritis. Dr. Thierry notes that ongoing and repeated NET formation, driven by inflammatory and clotting cycles, may worsen disease outcomes.
Investigating Microclot NETs Interactions in Long COVID
To explore whether microclots and NETs interact in Long COVID, the research teams led by Prof Pretorius and Dr Thierry joined forces. Their goal was to determine whether these two features of the blood might be linked in ways that help explain persistent symptoms.
Key Findings From the Analysis
Using imaging flow cytometry and fluorescence microscopy, the researchers conducted detailed measurements of microclots and NETs in the plasma of Long COVID patients and compared them to samples from healthy volunteers. They also evaluated NETs by examining proteic markers and circulating DNA.
Their study revealed several notable results:
- Biomarkers related to both microclots and NETs were significantly higher in Long COVID patients.
- Patients showed not only more microclots but also larger ones.
- The researchers identified a structural association between microclots and NETs in all subjects, which appeared far stronger in Long COVID patients.
"This finding suggests the existence of underlying physiological interactions between microclots and NETs that, when dysregulated, may become pathogenic," explains Dr. Thierry.
AI Tools Improve Diagnostic Accuracy
Artificial Intelligence methods, including machine learning, were added to the biomarker analysis. These tools allowed the researchers to clearly differentiate between Long COVID patients and healthy individuals. The algorithms also highlighted the most informative biomarker combinations, offering potential pathways for personalized care.
According to Prof Pretorius, the findings point to a significant buildup of microclots in Long COVID patients, likely supported by an overproduction of NETs: "This interaction could render microclots more resistant to fibrinolysis, promoting their persistence in circulation and contributing to chronic microvascular complications," she explains.
Implications for Treatment and Future Biomarkers
By identifying how NETs may help stabilize microclots, the study adds important insight into the biological processes underlying Long COVID. The results support the development of therapies aimed at reducing harmful clotting and inflammation.
The work also advances efforts to identify new biomarkers for diagnosing and monitoring post-viral conditions. As the researchers conclude, "The combination of advanced imaging techniques and machine learning confers methodological robustness and contributes significantly to the ongoing scientific discourse on post-viral syndromes," they conclude.
Reference: "Circulating Microclots Are Structurally Associated With Neutrophil Extracellular Traps and Their Amounts Are Elevated in Long COVID Patients" by Alain R. Thierry, Tom Usher, Cynthia Sanchez, Simone Turner, Chantelle Venter, Brice Pastor, Maxine Waters, Anel Thompson, Alexia Mirandola, Ekaterina Pisareva, Corinne Prevostel, Gert J. Laubscher, Douglas B. Kell and Etheresia Pretorius, 2 October 2025, Journal of Medical Virology.
DOI: 10.1002/jmv.70613
News
New Toothpaste Stops Gum Disease Without Harming Healthy Bacteria
Researchers have developed a targeted approach to combat periodontitis without disrupting the natural balance of the oral microbiome. The innovation could reshape how gum disease is treated while preserving beneficial bacteria. The human mouth [...]
Plastic Without End: Are We Polluting the Planet for Eternity?
The Kunming Montreal Global Biodiversity Framework calls for the elimination of plastic pollution by 2030. If that goal has been clearly set, why have meaningful measures that create real change still not been implemented? [...]
Scientists Rewire Natural Killer Cells To Attack Cancer Faster and Harder
Researchers tested new CAR designs in NK-92 cells and found the modified cells killed tumor cells more effectively, showing stronger anti-cancer activity. Researchers at the Ribeirão Preto Blood Center and the Center for Cell-Based [...]
New “Cellular” Target Could Transform How We Treat Alzheimer’s Disease
A new study from researchers highlights an unexpected player in Alzheimer’s disease: aging astrocytes. Senescent astrocytes have been identified as a major contributor to Alzheimer’s progression. The cells lose protective functions and fuel inflammation, particularly in [...]
Treating a Common Dental Infection… Effects That Extend Far Beyond the Mouth
Successful root canal treatment may help lower inflammation associated with heart disease and improve blood sugar and cholesterol levels. Treating an infected tooth with a successful root canal procedure may do more than relieve [...]
Microplastics found in prostate tumors in small study
In a new study, researchers found microplastics deep inside prostate cancer tumors, raising more questions about the role the ubiquitous pollutants play in public health. The findings — which come from a small study of 10 [...]
All blue-eyed people have this one thing in common
All Blue-Eyed People Have This One Thing In Common Blue Eyes Aren’t Random—Research Traces Them Back to One Prehistoric Human It sounds like a myth at first — something you’d hear in a folklore [...]
Scientists reveal how exercise protects the brain from Alzheimer’s
Researchers at UC San Francisco have identified a biological process that may explain why exercise sharpens thinking and memory. Their findings suggest that physical activity strengthens the brain's built in defense system, helping protect [...]
NanoMedical Brain/Cloud Interface – Explorations and Implications. A new book from Frank Boehm
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
Deadly Pancreatic Cancer Found To “Wire Itself” Into the Body’s Nerves
A newly discovered link between pancreatic cancer and neural signaling reveals a promising drug target that slows tumor growth by blocking glutamate uptake. Pancreatic cancer is among the most deadly cancers, and scientists are [...]
This Simple Brain Exercise May Protect Against Dementia for 20 Years
A long-running study following thousands of older adults suggests that a relatively brief period of targeted brain training may have effects that last decades. Starting in the late 1990s, close to 3,000 older adults [...]
Scientists Crack a 50-Year Tissue Mystery With Major Cancer Implications
Researchers have resolved a 50-year-old scientific mystery by identifying the molecular mechanism that allows tissues to regenerate after severe damage. The discovery could help guide future treatments aimed at reducing the risk of cancer [...]
This New Blood Test Can Detect Cancer Before Tumors Appear
A new CRISPR-powered light sensor can detect the faintest whispers of cancer in a single drop of blood. Scientists have created an advanced light-based sensor capable of identifying extremely small amounts of cancer biomarkers [...]
Blindness Breakthrough? This Snail Regrows Eyes in 30 Days
A snail that regrows its eyes may hold the genetic clues to restoring human sight. Human eyes are intricate organs that cannot regrow once damaged. Surprisingly, they share key structural features with the eyes [...]
This Is Why the Same Virus Hits People So Differently
Scientists have mapped how genetics and life experiences leave lasting epigenetic marks on immune cells. The discovery helps explain why people respond so differently to the same infections and could lead to more personalized [...]
Rejuvenating neurons restores learning and memory in mice
EPFL scientists report that briefly switching on three “reprogramming” genes in a small set of memory-trace neurons restored memory in aged mice and in mouse models of Alzheimer’s disease to level of healthy young [...]