Cardiovascular disease continues to be the leading cause of death worldwide. But advances in heart-failure therapeutics have stalled, largely due to the difficulty of delivering treatments at the cellular level. Now, a UC Berkeley-led team of researchers may have solved this delivery bottleneck, potentially opening the door to novel, lifesaving treatments.
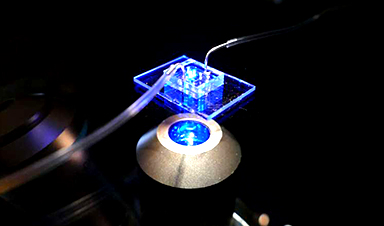
At the core of their new approach is a human cardiac microphysiological system (MPS)—also known as a heart-on-a-chip—that provides a miniaturized model of the human heart, complete with 3D micromuscles. Such devices consist of microfluidic channels, less than the width of a human hair, lined with living human cells. By controlling the fluid flow and other elements, researchers can mimic aspects of the heart’s physiology.
Using their heart-on-a-chip, researchers from UC Berkeley, the Gladstone Institutes and UCSF were able to discover a lipid nanoparticle that could penetrate the dense heart muscle and efficiently deliver its cargo of therapeutic messenger RNA (mRNA) into heart muscle cells, or cardiomyocytes.
Their findings are published in Nature Biomedical Engineering.
Lipid nanoparticles are tiny, spherical particles made of fats that encapsulate therapeutic agents. They are considered the most clinically advanced nonviral transport system for delivering mRNA in gene editing therapies and in vaccines, including the Pfizer-BioNTech and Moderna COVID-19 shots.
However, successfully delivering mRNA to cardiomyocytes hinges on something called endosomal escape, long seen as a challenge in this field. The endosome acts as a cell’s sorting station, and if the therapeutic agent gets stuck there, it will start to degrade. To be effective, the lipid nanoparticle must exit the endosome and enter the cell’s cytoplasm, where it can distribute its mRNA cargo for maximum therapeutic effect.
To tackle this problem, the researchers synthesized lipid nanoparticles with a novel acid-degradable polyethylene glycol coating, with the idea that it could easily diffuse through heart tissue and still leave the endosome. Using their heart-on-a-chip, they then tested different iterations to identify the most effective version for delivering the gene-editing therapy to cardiomyocytes. Later, they tested these same lipid nanoparticles on mouse hearts and recorded similar, positive results.
According to Kevin Healy, co-principal investigator of the study, the researchers’ organ-on-a-chip approach also could allow scientists to more accurately predict test results on live organisms and accelerate advances in mRNA cardiac therapies. The key, he said, is the model’s ability to replicate the complex 3D cellular environments of microtissues better than simple 2D models, which typically consist of a single layer of cells grown in a petri dish.
“Our framework enables faster, animal-sparing identification of effective lipid nanoparticles for safely delivering these therapies,” said Healy, professor of bioengineering and of materials science and engineering at UC Berkeley. “So, by using organ-on-a-chip models to predict heart-targeted delivery and safety, we can potentially accelerate programs for heart failure therapeutics, cardioprotective factors and gene correction, while reducing time and cost to translation.”
More information: Gabriel Neiman et al, A microphysiological system for screening lipid nanoparticle−mRNA complexes predicts in vivo heart transfection efficacy, Nature Biomedical Engineering (2025). DOI: 10.1038/s41551-025-01523-4
Journal information: Nature Biomedical Engineering
News
A Virus Designed in the Lab Could Help Defeat Antibiotic Resistance
Scientists can now design bacteria-killing viruses from DNA, opening a faster path to fighting superbugs. Bacteriophages have been used as treatments for bacterial infections for more than a century. Interest in these viruses is rising [...]
Sleep Deprivation Triggers a Strange Brain Cleanup
When you don’t sleep enough, your brain may clean itself at the exact moment you need it to think. Most people recognize the sensation. After a night of inadequate sleep, staying focused becomes harder [...]
Lab-grown corticospinal neurons offer new models for ALS and spinal injuries
Researchers have developed a way to grow a highly specialized subset of brain nerve cells that are involved in motor neuron disease and damaged in spinal injuries. Their study, published today in eLife as the final [...]
Urgent warning over deadly ‘brain swelling’ virus amid fears it could spread globally
Airports across Asia have been put on high alert after India confirmed two cases of the deadly Nipah virus in the state of West Bengal over the past month. Thailand, Nepal and Vietnam are among the [...]
This Vaccine Stops Bird Flu Before It Reaches the Lungs
A new nasal spray vaccine could stop bird flu at the door — blocking infection, reducing spread, and helping head off the next pandemic. Since first appearing in the United States in 2014, H5N1 [...]
These two viruses may become the next public health threats, scientists say
Two emerging pathogens with animal origins—influenza D virus and canine coronavirus—have so far been quietly flying under the radar, but researchers warn conditions are ripe for the viruses to spread more widely among humans. [...]
COVID-19 viral fragments shown to target and kill specific immune cells
COVID-19 viral fragments shown to target and kill specific immune cells in UCLA-led study Clues about extreme cases and omicron’s effects come from a cross-disciplinary international research team New research shows that after the [...]
Smaller Than a Grain of Salt: Engineers Create the World’s Tiniest Wireless Brain Implant
A salt-grain-sized neural implant can record and transmit brain activity wirelessly for extended periods. Researchers at Cornell University, working with collaborators, have created an extremely small neural implant that can sit on a grain of [...]
Scientists Develop a New Way To See Inside the Human Body Using 3D Color Imaging
A newly developed imaging method blends ultrasound and photoacoustics to capture both tissue structure and blood-vessel function in 3D. By blending two powerful imaging methods, researchers from Caltech and USC have developed a new way to [...]
Brain waves could help paralyzed patients move again
People with spinal cord injuries often lose the ability to move their arms or legs. In many cases, the nerves in the limbs remain healthy, and the brain continues to function normally. The loss of [...]
Scientists Discover a New “Cleanup Hub” Inside the Human Brain
A newly identified lymphatic drainage pathway along the middle meningeal artery reveals how the human brain clears waste. How does the brain clear away waste? This task is handled by the brain’s lymphatic drainage [...]
New Drug Slashes Dangerous Blood Fats by Nearly 40% in First Human Trial
Scientists have found a way to fine-tune a central fat-control pathway in the liver, reducing harmful blood triglycerides while preserving beneficial cholesterol functions. When we eat, the body turns surplus calories into molecules called [...]
A Simple Brain Scan May Help Restore Movement After Paralysis
A brain cap and smart algorithms may one day help paralyzed patients turn thought into movement—no surgery required. People with spinal cord injuries often experience partial or complete loss of movement in their arms [...]
Plant Discovery Could Transform How Medicines Are Made
Scientists have uncovered an unexpected way plants make powerful chemicals, revealing hidden biological connections that could transform how medicines are discovered and produced. Plants produce protective chemicals called alkaloids as part of their natural [...]
Scientists Develop IV Therapy That Repairs the Brain After Stroke
New nanomaterial passes the blood-brain barrier to reduce damaging inflammation after the most common form of stroke. When someone experiences a stroke, doctors must quickly restore blood flow to the brain to prevent death. [...]
Analyzing Darwin’s specimens without opening 200-year-old jars
Scientists have successfully analyzed Charles Darwin's original specimens from his HMS Beagle voyage (1831 to 1836) to the Galapagos Islands. Remarkably, the specimens have been analyzed without opening their 200-year-old preservation jars. Examining 46 [...]