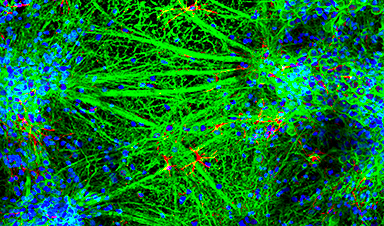
You can 3D print nearly anything: rockets, mouse ovaries, and for some reason, lamps made of orange peels. Now, scientists at Monash University in Melbourne, Australia, have printed living neural networks composed of rat brain cells that seem to mature and communicate like real brains do.
Researchers want to create mini-brains partly because they could someday offer a viable alternative to animal testing in drug trials and studies of basic brain function. At the start of 2023, the US Congress passed an annual spending bill pushing scientists to reduce their use of animals in federally funded research, following the signing of the US Food and Drug Administration’s Modernization Act 2.0, which allowed high-tech alternatives in drug safety trials. Rather than testing new drugs on thousands of animals, pharmaceutical companies could apply them to 3D-printed mini-brains—in theory. There are still complexities to iron out before this moves from proof of concept to standard lab practice.
3D-printing is just one entry in the race to build a better mini-brain. One existing option is culturing a single layer of neurons in a petri dish, guiding cells to grow over recording electrodes. Growing the tissue around electrodes is convenient for running experiments, but it comes at the cost of biological realism. (Brains aren’t flat.) To get closer to the brain’s true structure, researchers can instead coax a bunch of stem cells to organize themselves into 3D tissues called organoids—but can’t fully control how they grow.
The Monash team tried to split the difference. With 3D-printing, researchers can culture cells in specific patterns on top of recording electrodes, granting them a degree of experimental control normally reserved for flat cell cultures. But because the structure is soft enough to allow cells to migrate and reorganize themselves in 3D space, it gains some of the advantages of the organoid approach, more closely mimicking the structure of normal tissue. “You kind of have the best of both worlds,” says Michael Moore, professor of biomedical engineering at Tulane University in New Orleans, Louisiana, who was not involved in this study.
Led by materials science and engineering professor John Forsythe, the Monash team described their experiment in June in Advanced Healthcare Materials. Just like how inkjet printers funnel ink from cartridges to a piece of paper, Forsythe’s team printed neural structures by squeezing “bioink”—rat brain cells suspended in a gel—out of a nozzle and into a scaffold. They built their neural networks by crosshatching layer by layer, stacking eight vertical layers alternating between bioinks with and without cells. (These bioinks were extruded from different cartridges, like switching between black and color.) This structure gave cells ready access to the gel’s nutrients while mimicking alternating between gray and white matter in the cortex, where gray matter contains neuron cell bodies and white matter contains the long axons connecting them.
A tiny array of microelectrodes underneath the cells recorded electrical activity in the gel surrounding the cells, while other electrodes directly stimulated the neurons and recorded their responses. Using a fluorescent dye to visualize the movement of calcium ions under a microscope, the team was able to watch the cells chemically communicate. “They behaved as we would expect,” Forsythe says. “There were no surprises.”
That starts by making sure you don’t kill the cells when you print them. When standard 3D-printers work with plastic filaments, they melt the plastic to make it moldable, heating it up to temperatures far beyond those found in the human body. This is a nonstarter for neurons, extremely finicky cells that can survive only in carefully calibrated gels that closely replicate properties of squishy, body-temperature brains. “Making a gel that is as soft as the brain, but that you can still print through a 3D-printer, is really hard,” says Moore.
“It’s important not to kill the cells. But with neurons, it’s really important not to kill your electrical activity,” adds Stephanie Willerth, a professor of biomedical engineering at the University of Victoria in Canada, who was not involved in this study. Earlier versions of 3D-printed neural tissue often excluded glial cells, which help maintain a welcoming environment for their sensitive neuron neighbors. Without them, “neurons still have some electrical activity, but it’s not going to fully replicate what you see in the body,” she says.
Willerth thinks the new experiment is promising. These neural networks were made of rat cells, but “it’s a proof of concept showing that you can eventually do this with human cells,” Willerth says. Still, future experiments will need to replicate this level of function in human cells before these neural network models can be used in translational research and medicine.
There is also a scaling issue. The tissues printed in the Monash experiment contained a few thousand neurons per square millimeter, amounting to a couple hundred thousand cells in each 8 x 8 x 0.4 mm structure. But the human brain has about 16 billion neurons in the cortex alone, not to mention billions more glial cells.
As Moore points out, 3D-printing such delicate tissue is relatively slow, even when the final product is tiny. More work needs to be done before this precise but sluggish technique can be scaled up from academic research labs to Big Pharma, where companies are often testing dozens of drugs at once. “It’s not impossible,” Moore says. “It’s just going to be difficult.” (AxoSim, a neuroengineering startup cofounded by Moore, has already started building 3D models of human neurons and peripheral nerves for commercial drug testing.)
While this technology has the potential to replace animals in many research settings, from basic neuroscience to commercial drug development, scientists may be slow to make the switch. Often, Moore finds, scientists like him are “stuck in our ways,” reluctant to spend the time, money, and effort required to move away from tried-and-true animal models. “Convincing scientists to abandon those approaches for fancy engineered tissue is going to take time,” he says, “but I’m very optimistic that we will gradually reduce the number of animal studies.”
When dealing with brainlike structures, one can’t help but think about … thinking. While researchers don’t yet have good ways of defining or measuring consciousness in lab-grown neural networks, “there are possibilities of creating living artificial neural networks using this technique,” Forsythe says. Last year, a team of scientists managed to use electrical stimulation and recording to link a petri dish filled with neurons to a computer, where they appeared to learn to play Pong in about five minutes. Some, like Thomas Hartung at Johns Hopkins University, believe that 3D neural networks will merge with AI to produce “organoid intelligence” that researchers will someday be able to harness for biological computing.
In the more immediate future, Forsythe and his team hope to see how their printed neural networks fare under stress. Understanding the extent to which these tissues can regenerate after suffering cellular damage will uncover important clues about the brain’s ability to heal from trauma. Someday, Forsythe believes, people may be able to receive personalized treatments for neurodegenerative diseases and other brain injuries, informed by models of their own neural tissue. Willerth envisions hospitals hosting 3D-printing suites, where future clinicians will be able to use patient biopsies to print tissues that can be used to test whether a given drug will actually work for them. “It sets the stage for that kind of personalized medicine,” she says. “Papers like this will drive it forward.”
Engineering personalized brain treatments will be no small feat, but the research community is well on its way. “We’re inching our way closer to being able to do experiments that don’t require animals in the most complex organ that we know of,” says Moore. “Perhaps the most complex structure in the entire universe.”
News
Most Plastic in the Ocean Is Invisible—And Deadly
Nanoplastics—particles smaller than a human hair—can pass through cell walls and enter the food web. New research suggest 27 million metric tons of nanoplastics are spread across just the top layer of the North [...]
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]