Several antivirals, including remdesivir, Paxlovid, molnupiravir, and monoclonal antibodies like tixagevimab and cilgavimab, have been repurposed to treat the coronavirus disease 2019 (COVID-19) or received emergency use authorization (EUA). Antimalarial and antiparasitic drugs like ivermectin, hydroxychloroquine, and chloroquine have also been investigated for their potential activity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
A recent review published in the journal Acta Pharmacologica Sinica discusses the cardiovascular adverse effects associated with antiviral drugs used to treat COVID-19.
About the virus
SARS-CoV-2 is a single-stranded ribonucleic acid (RNA) virus enclosed in a protein envelope comprising the membrane, spike, and envelope proteins. Viral RNA is stored within the nucleocapsid, comprised of the nucleocapsid protein.
The SARS-CoV-2 spike protein recognizes and subsequently binds to the angiotensin-converting enzyme 2 (ACE2) receptor present on the surface of the host cell. The S1 subunit of the spike protein consists of an N-terminal domain (NTD) and receptor-binding domain (RBD).
RBD-ACE2 binding causes the S2 subunit to dissociate from the ACE2 molecule, which subsequently causes the virus to transition from a pre to post-fusion state. Thereafter, the virus and host cell membranes fuse together, thereby allowing viral entry into the cell.
ACE2 and cardiovascular adverse effects
ACE2 regulates the vasoactive effects of ACE, which converts angiotensin I to angiotensin II, a potent vasoconstrictor and pro-inflammatory agent. Angiotensin II induces hyperinflammation due to the dysregulated release of cytokines, leading to severe tissue damage and multi-organ failure, which is often characteristic of severe COVID-19.
Pre-pandemic antivirals and cardiovascular effects
Idoxuridine was the first antiviral approved in 1963 for feline herpesvirus-1 eye infections; since then, 37 antivirals have been approved to treat a wide range of infections caused by the human immunodeficiency virus (HIV), hepatitis B virus (HBV), cytomegalovirus (CMV), influenza virus, respiratory syncytial virus (RSV), and hepatitis C virus (HCV).
Among the drugs used to treat HIV include protease inhibitors like lopinavir/ritonavir, which increase lipid levels in the blood, liver, and heart, in addition to weakening heart pumping activity. Endothelial damage has also been observed, which may cause atherosclerosis with its cardiovascular sequelae. Interferon-α, which is used in the treatment of multiple viral infections and cancers, has also been associated with adverse cardiac effects.
Remdesivir
Remdesivir is a prodrug that converts to an analog of the nucleotide adenosine, thereby disrupting viral replication. The vasodilation activity of adenosine can induce the release of catecholamines like epinephrine, thereby increasing the risk of ventricular tachycardia, ventricular fibrillation, and atrial fibrillation.
When administered intravenously, remdesivir can trigger QT prolongation and the potentially deadly arrhythmia torsade de pointes. Thus, continuous heart monitoring is essential for COVID-19 patients being treated with remdesivir, especially those with pre-existing cardiac disease or electrolyte abnormalities.
Paxlovid
Paxlovid, which consists of nirmatrelvir and ritonavir, may cause bradycardia and sinus dysfunction. However, it remains unclear which component of Paxlovid is responsible and what mechanisms are involved in this adverse side effect.
The toxicity of Paxlovid, when combined with tacrolimus, an immunosuppressant, has been reported in several cases. Paxlovid may also increase the risk of bleeding when used in combination with ticagrelor, warfarin, or rivaroxaban.
Paxlovid may also interact with other drugs to cause skeletal muscle breakdown and myopathy.
Molnupiravir
Esterases in host plasma activate molnupiravir to its active antiviral nucleoside analog EIDD-1931. Molnupiravir can increase oxidant stress, which may cause tissue damage. However, like Paxlovid, the use of molnupiravir can reduce the risk of severe COVID-19, particularly among those with diabetes and patients 65 years of age and older.
Other drugs
Hydroxychloroquine (HCQ) acidifies intracellular endosomes and affects the viral life cycle at multiple stages. Its therapeutic effects may be synergistic with those of azithromycin.
Nevertheless, both HCQ and azithromycin can cause prolonged QTc or cardiac arrhythmias. Thus, the combination of these drugs may not be ideal for severe COVID-19 or patients at an increased risk of QT prolongation.
Ivermectin
Ivermectin inhibits interactions between the virus and host cell, thereby preventing nuclear transport of viral proteins. However, preclinical data suggests the accumulation of ivermectin in the heart and inhibition of potassium currents. Patients with COVID-19 who are treated with ivermectin should be monitored for arrhythmias or QT prolongation.
Antibodies
Both monoclonal antibodies (mAbs) and plasma have been used to treat COVID-19. Cardiac arrhythmias have been reported with mAb, particularly following treatment with tixagevimab or cilgavimab.
The combination of cilgavimab and tocilizumab may cause thromboembolic events. Hypertension is most commonly reported with mAbs like casirivimab and imdevimab, bamlanivimab alone or with etesevimab, and sotrovimab.
Conclusions
The potential cardiovascular side effects of COVID-19 therapeutics must be carefully considered before prescribing these agents to high-risk patients. Despite reported observations of cardiotoxicity, additional studies are needed to differentiate the cardiovascular effects of SARS-CoV-2 infection from those of antivirals.
Future antiviral drug development assisted with the newest artificial intelligence platform may improve the accuracy to predict the structures of biomolecules of antivirals and therefore to mitigate their associated cardiovascular adversities.”
- Chen, E., & Xi, L. (2024). Cardiovascular adverse effects of antiviral therapies for COVID-19: Evidence and plausible mechanisms. Acta Pharmacologica Sinica. doi:10.1038/s41401-024-01382-w.
News
Nanocrystals Carrying Radioisotopes Offer New Hope for Cancer Treatment
The Science Scientists have developed tiny nanocrystal particles made up of isotopes of the elements lanthanum, vanadium, and oxygen for use in treating cancer. These crystals are smaller than many microbes and can carry isotopes of [...]
New Once-a-Week Shot Promises Life-Changing Relief for Parkinson’s Patients
A once-a-week shot from Australian scientists could spare people with Parkinson’s the grind of taking pills several times a day. The tiny, biodegradable gel sits under the skin and releases steady doses of two [...]
Weekly injectable drug offers hope for Parkinson’s patients
A new weekly injectable drug could transform the lives of more than eight million people living with Parkinson's disease, potentially replacing the need for multiple daily tablets. Scientists from the University of South Australia [...]
Most Plastic in the Ocean Is Invisible—And Deadly
Nanoplastics—particles smaller than a human hair—can pass through cell walls and enter the food web. New research suggest 27 million metric tons of nanoplastics are spread across just the top layer of the North [...]
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
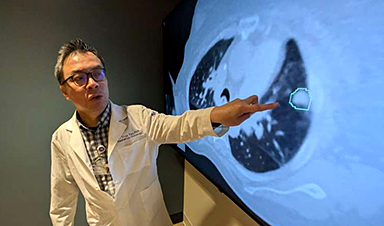
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]