Some of the world’s most important discoveries – penicillin, vulcanized rubber and Velcro, to name a few – were made by accident. In fact, it’s been said that upward of half of all scientific discoveries are by chance.
Add vortex ring freezing to that long list of “accidents.”
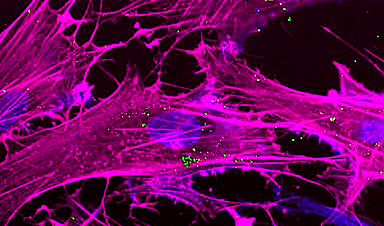
Duo An, a doctoral student in the labs of both professor Dan Luo and assistant professor Minglin Ma, in the Department of Biological and Environmental Engineering, was an undergraduate from China doing an internship at Cornell when he stumbled upon a phenomenon that has the potential to greatly improve cell-free protein production and cell delivery, particularly for Type 1 diabetes patients.
A group headed by Luo and Ma has published the paper, “Mass production of shaped particles through vortex ring freezing,” which was released online Aug. 4 in Nature Communications. An is lead author.
Vortex rings are ubiquitous in nature – a mushroom cloud of smoke is one example – and the ring’s evolution exhibits a rich spectrum of complicated geometries, from spherical to teardrop to toroidal (doughnut-shaped). The researchers used these features to control and mass produce inorganic and organic particles via an electrospraying process, whereby a multitude of vortex ring-derived particles (VRPs) can be produced, then frozen at precise time points. The group reported they could produce 15,000 rings per minute via electrospraying.
They found controlling the shape and speed of the spray, as well as the speed of the chemical reaction, can yield different structures.
“We can tune both of these timescales, and control at which stage we can freeze the structure, to get the results we want,” An said.
Recent News
The Global Nanomedicine Market: Key Players and Emerging Technologies in Healthcare
This article provides an overview of the global nanomedicine market, highlighting key players, emerging technologies, and the challenges and opportunities that influence its growth and commercialization in the healthcare [...]
Scientists Have Discovered Toxic “Forever Chemicals” in Bottled Water
Scientists have found toxic PFAS in drinking water samples from around the world, with higher levels in tap water from China compared to the UK. Boiling water or using [...]
Urban Microbes Are Eating Disinfectants – Are We Fueling a New Health Threat?
New research reveals that microbes in urban environments are evolving to withstand the very cleaning agents designed to eliminate them. The study also uncovers new strains in Hong Kong, [...]
Startling Study Shows High-Potency Cannabis Alters DNA
The study shows that frequent use of high-potency cannabis alters DNA, affecting genes related to energy and immune function. These changes differ between those with and without psychosis, suggesting cannabis use could [...]
New nanotherapy targets artery inflammation in cardiovascular disease
Inflammation of the arteries is a primary precursor and driver of cardiovascular disease—the No. 1 killer of people in the United States. This inflammation is associated with the buildup [...]
Revolutionary Nanoparticle Therapy for Prostate Cancer
A groundbreaking research effort involving teams from the University of Virginia, Mount Sinai, the University of Michigan, the University of Texas, and others has displayed the clinical efficacy of an innovative [...]
Antibody engineering drives innovation in drug development
Monoclonal antibodies (mAbs) are used to prevent, detect, and treat a broad spectrum of non-communicable and communicable diseases. Over the past few years, the market for mAbs has grown [...]
Breakthrough Study Reveals How Bladder Cancer Starts and Spreads
Researchers found that DNA mutations from antiviral enzymes and chemotherapy fuel early bladder cancer, while abnormal circular DNA in tumor cells drives resistance to therapy. These discoveries open new therapeutic avenues. [...]
AI and Quantum Mechanics Accelerate Drug Discovery
A recent article published in the Journal of Chemical Information and Modeling researchers at Southern Methodist University (SMU) have developed SmartCADD, an open-source [...]
Targeting ‘undruggable’ diseases: Researchers reveal new levels of detail in targeted protein degradation
Researchers at the University of Dundee have revealed in the greatest detail yet the workings of molecules called protein degraders which can be deployed to combat what have previously [...]
Revolutionizing Virology: AI Discovers Over 160,000 New RNA Viruses
Largest discovery of new virus species sheds light on the hidden virosphere. Artificial intelligence (AI) has been used to reveal details of a diverse and fundamental branch of life living right under [...]
Cardiac Crisis: COVID-19 Doubles Risk of Heart Attacks, Strokes, and Death
Research indicates that COVID-19 survivors face doubled risks of severe cardiac events for years after recovery, especially if hospitalized. People with A, B, or AB blood types are particularly vulnerable, highlighting [...]
AI steps into science limelight with Nobel wins
For long periods of its history, artificial intelligence has lurked in the hinterland of science, often unloved and unfunded—but two Nobel prizes in one week suggest its time in [...]
MIT Scientists Shed New Light on the Critical Brain Connections That Define Consciousness
A new study provides further evidence that consciousness depends on communication between the brain’s sensory and cognitive regions in the cortex. Our brains are constantly making predictions about our [...]
Common Chemicals Found in Shampoo and Plastic Could Be Quietly Disrupting Your Heart’s Rhythm
UC study of Fernald data links environmental phenols to heart toxicities Environmental phenols are present in numerous everyday consumer products, serving as preservatives in packaged foods, parabens in shampoos, [...]
Revolutionary Brain Tech Offers New Hope for Stroke and Injury Recovery
University of Pittsburgh researchers report that deep brain stimulation (DBS) can effectively enhance motor functions in individuals with arm and hand paralysis due to brain injuries, with promising results [...]
NIH Scientists Discover Gene Responsible for Rare Eye Disease
Findings supported by the NIH pave the way for the development of genetic testing, clinical trials, and therapies. Researchers at the National Institutes of Health (NIH) and their collaborators have discovered [...]
Alzheimer’s Breakthrough: Synthetic THC Pill Proves Effective in Clinical Trial
Patients tolerated synthetic THC (dronabinol) well, without the adverse effects commonly associated with existing Alzheimer’s agitation medications. A study conducted by researchers from Johns Hopkins University School of Medicine and Tufts University School of [...]
The Future of Rare Disease Treatment with Precision Medicine
Understanding rare diseases Rare diseases affect less than 5 people out of 10,000. However, this still amounts to about 7% of the world’s population, with over 10,000 such conditions. [...]
Doctors issue warning for upcoming ‘tripledemic
The term ‘tripledemic’ has hit headlines this week as the NHS begins its Covid and fluvaccine roll-out for vulnerable adults. As the cold weather sets in, many of us have experienced a decline [...]
The FDA approved a gel that can stop bleeding from wounds in seconds
Aug 15 (Reuters) - The U.S. Food and Drug Administration has cleared Cresilon's gel to quickly control bleeding, the privately held company said on Thursday, potentially giving emergency medical [...]
High levels of microplastics found in prostate tumors, possibly linked to take-out food
The presence of microplastics in prostate tumors points to potential health risks, and researchers are calling for urgent studies to explore how take-out food may be driving this exposure. [...]
AI outperforms radiologists in brain tumor diagnosis
As artificial intelligence advances, its uses and capabilities in real-world applications continue to reach new heights that may even surpass human expertise. In the field of radiology, where a correct [...]
Breakthrough Study Reveals Molecular Clues to Dementia Origins
Work could lead to the discovery of new therapeutic targets. For the first time, researchers have identified “molecular markers” linked to degeneration—detectable changes in cells and their gene-regulating networks—that [...]
Better than blood tests? Nanoparticle potential found for assessing kidneys
In a study published July 29 in Advanced Materials, University of Texas at Dallas researchers found that X-rays of the kidneys using gold nanoparticles as a contrast agent might be [...]
Greener nanomaterials could transform how our everyday stuff is made
Tiny nanoparticles are at the forefront of materials science—with special properties that make them great at absorbing light in solar panels, cleaning wastewater, and delivering drugs precisely. Some nanoparticles take the form of sheets [...]
AI could predict breast cancer risk via ‘zombie cells’
Women worldwide could see better treatment with new AI technology, which enables better detection of damaged cells and more precisely predicts the risk of getting breast cancer, [...]
Through the eyes of a cat – biomimicry of feline eyes may revolutionize robotic vision
In a recent study published in the journal Science Advances, researchers leveraged crucial aspects of feline eyes, particularly their tapetum lucidum and vertically elongated pupils (VP), to develop a monocular [...]
New Alzheimer’s Therapy Shows Remarkable Results in Animal Trials
A study from TUM demonstrates a promising therapeutic approach. Researchers at the Technical University of Munich (TUM) have made promising advances in preventing Alzheimer’s by developing a new therapeutic strategy. Their approach focuses [...]
Rewriting Cancer’s Blueprint: New Study Challenges Old Theories
A new study argues for a revised clonal evolution model of cancer, incorporating genetic and non-genetic factors to improve understanding and treatment. Like all living organisms, cancer cells are [...]
Microplastics Everywhere: Experts Demand Worldwide Treaty Before It’s Too Late
A new report calls for global action on plastic pollution, urging reductions in plastic production and microplastic emissions. Researchers stress the importance of addressing plastic pollution through both scientific [...]
Blood tests could soon predict your risk of Alzheimer’s
Scientists are closing in on biomarkers that reflect the progression of Alzheimer’s disease and could improve treatments. Like many Alzheimer’s researchers, neurologist Randall Bateman is not prone to effusiveness, [...]
Recharging mitochondria—nanoflowers offer a new way to simulate energy production
When we need to recharge, we might take a vacation or relax at the spa. But what if we could recharge at the cellular level, fighting against aging and [...]
Revealing the Invisible: Living Cells Can Be Seen With Infrared Light
IST’s new infrared microscopy technique allows for the detailed imaging of biomolecules in cells, supporting advancements in biotechnology and cellular therapies. In an effort to advance biotechnology innovations, scientists [...]
3,600+ Chemicals From Food Packaging Found in Human Bodies
A recent review has uncovered the widespread presence of food contact chemicals (FCC) in humans, identifying 3,601 chemicals used in food packaging and related products found in the human [...]
CREME: A New AI-Powered Virtual Lab to Help Cure Genetic Diseases
CREME, an AI-powered virtual lab, developed at Cold Spring Harbor Laboratory, offers a revolutionary approach to genetic research by simulating CRISPR interference (CRISPRi). This tool enables scientists to perform [...]
New Research Reveals That Cannabis Can Reverse Brain Aging
Researchers in Bonn examine how treatment with tetrahydrocannabinol affects the mTOR metabolic pathway. A low-dose, long-term administration of cannabis has been shown to not only reverse aging processes in the brain [...]
Cardiovascular risks of COVID-19 antivirals
Several antivirals, including remdesivir, Paxlovid, molnupiravir, and monoclonal antibodies like tixagevimab and cilgavimab, have been repurposed to treat the coronavirus disease 2019 (COVID-19) or received emergency use authorization (EUA). [...]
Your eyes could reveal the first signs of many diseases
Melissa, a 30-year-old educator, came to the emergency department with a sudden onset of double vision. She had not been in an accident or suffered any trauma and had [...]
New insight into the causes of autoimmune diseases
Autoimmune diseases are widespread and notoriously difficult to treat. In part, this is because why the immune system attacks its own tissues in patients with these conditions remains poorly [...]
COVID-19 reduces male fertility by affecting semen quality and hormone levels
In a recent study published in the journal PLOS ONE, researchers conducted a systematic review and meta-analyzed data on the impact of coronavirus disease 2019 (COVID-19) on male reproductive hormones [...]
New Alzheimer’s Study Unlocks the Secrets of Aging Brain Cells
New method uses patient-derived neurons to effectively simulate late-onset Alzheimer’s and identify possible treatments. Researchers at Washington University School of Medicine in St. Louis have created a technique to explore the impact of [...]
Lockdowns prematurely aged teenagers’ brains, study suggests
Teenage girls' brains may have prematurely aged by up to four years during the Covid pandemic, an American study suggests. Adolescent boys weren't immune either with their brain's also [...]
Long COVID Still a Mystery: Routine Labs Show No Reliable Biomarkers
Routine lab tests are not reliable for diagnosing Long COVID, according to a new study. The research found that no clinical lab values could serve as biomarkers, highlighting the [...]
Tiny magnetic robots could treat bleeds in the brain
Researchers have created nanoscale robots which could be used to manage bleeds in the brain caused by aneurysms. The development could enable precise, relatively low-risk treatment of brain aneurysms, which [...]
Turning Mosquito Spit Into a Weapon Against the West Nile Virus and Other Deadly Diseases
Anita Saraf investigates mosquito saliva to understand how viruses like dengue and West Nile are transmitted, using mass spectrometry to identify potential targets for vaccines and treatments. You might [...]
Ethics in Nanomedicine: Key Issues and Principles
Nanomedicine, a branch of nanotechnology, is revolutionizing healthcare by enabling the manipulation of materials at the nanoscale to diagnose, treat, and prevent diseases. Unlike traditional treatments, nanoparticles (NPs) are [...]
A call for robust H5N1 influenza preparedness and response
As the global threat of H5N1 influenza looms with outbreaks across species and continents including the U.S., three international vaccine and public health experts say it is time to [...]
Mucosal COVID-19 boosters outperform mRNA shots in preventing upper airway infections
In a recent study published in Nature Immunology, a team of researchers from the United States used non-human primate models to compare the protection conferred by an intramuscular booster dose [...]
How Space Travel Really Changes Astronauts – From the Inside Out
International team reveals previously unknown effects on physiology that could shape the future of long-duration space missions. Researchers have discovered significant changes in the gut microbiome due to spaceflight, [...]

































Leave A Comment