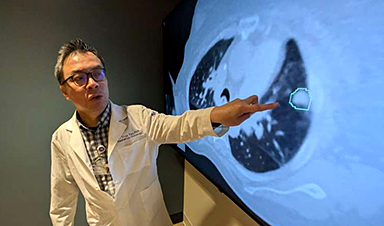
Sensors built with a new manufacturing approach are capable of recording activity deep within the brain from large populations of individual neurons—with a resolution of as few as one or two neurons—in humans as well as a range of animal models, according to a study published in the Jan. 17, 2024 issue of the journal Nature Communications.
The approach is unique in several ways. It relies on ultra-thin, flexible and customizable probes, made of clinical-grade materials, and equipped with sensors that can record extremely localized brain signals. Because the probes are much smaller than today’s clinical sensors, they can be placed extremely close to one another, allowing for high-resolution sensing in specific areas at unprecedented depths within the brain.
Right now, the probes can record with up to 128 channels, while the state of the art in today’s clinical probes is only eight to 16 channels. In the future, the innovative manufacturing approach the researchers developed can expand the number of channels to thousands per probe, dramatically enhancing physicians’ ability to acquire, analyze and understand brain signals at a higher resolution.
This technology is a first step towards wireless monitoring of patients with treatment-resistant epilepsy for extended periods of time–up to 30 days–as they go about their daily lives. Beyond treatment-resistant epilepsy, the potential applications are much broader, including helping people with Parkinson’s disease, movement disorders, obsessive-compulsive disorder, obesity, treatment-resistant depression, high-impact chronic pain and other disorders.

While the Nature Communications paper reports brain-recording data only, the system has been developed to both record brain activity and provide electrical stimulation to precise locations. In fact, the team is building on previous—and ongoing—work that uses this scalable, thin-film manufacturing approach to create brain-computer interfaces that record activity and deliver therapeutic electrical stimulation to the surface of the brain cortex.
The probes are monolithic, meaning that their individual components are layered on top of one another to create a single, cohesive unit, and do not require manual assembly of additional wires to conduct recordings.
The new recording system is both extremely customizable and scalable to manufacture, thanks to thin-film technology derived from the semiconductor and digital-display screen industries. As such, the probes are extremely compact—15 microns thick, or about 1/5th the thickness of a human hair—minimizing the differences between the material properties of the probe and the brain.
“We developed an entirely different manufacturing method for thin-film electrodes that can reach deep brain structures—at a depth that is necessary for therapeutic reasons—enabling reproducible, customizable, and high-throughput production of electrodes but with a high spatial resolution and channel count despite a thinner electrode body,” said UC San Diego electrical engineering professor Shadi Dayeh, the corresponding author on the new paper.
“Additionally, the electrode insertion is compatible with existing surgical techniques in the operating room, lowering the barrier for their adoption in clinical procedures.”
The design, manufacture, experimental testing and analysis of results from this system was performed by a cross-disciplinary team of engineers, surgeons, and medical researchers from UC San Diego; Harvard Medical School and Massachusetts General Hospital; and Oregon Health and Science University.
Dayeh advises two of the three first authors on the paper: UC San Diego postdoctoral researcher Keundong Lee and UC San Diego graduate student researcher Yun Goo Ro. Angelique C. Paulk, also a first author, is a researcher at Massachusetts General Hospital and Harvard Medical School in a group led by neurologist Dr. Sydney Cash.
Toward a 30-day wireless brain-recording system
The kind of system researchers developed is needed in order to identify the very specific regions of the brain that are triggering seizures caused by treatment-resistant epilepsy. To meet this goal, the team is working toward their vision of a brain-monitoring system with sensors both inserted deep within the brain and sensors on the surface of the brain.
These sensors will communicate wirelessly with a small computer system in a wireless cap, which a person could wear for extended periods of time. This cap would provide wireless power and the computational infrastructure to capture the brain signals being recorded from a person’s brain for 30 days.
“We are currently focused on applying the technology to patients with treatment-resistant epilepsy. The ultimate goal is to advance the system and related required technologies by 2026 to give patients access to a wireless system that allows them to move freely within the hospital environment and then at home, without being tethered to any machinery, while cortical and deep brain structures are monitored continuously for up to 30 days,” Dayeh said.
The system is called the UC San Diego Micro-stereo-electro-encephalography (µSEEG). The technology that is used to create the device can be manufactured at high volume and low cost because it is derived from existing technologies to manufacture digital display screens, an approach that was originally created by the semiconductor industry. This unique manufacturing process also allows for a series of unique features for these depth electrodes (see sidebar).
Experimental subjects
In the new paper, the team reports the functioning of the new system in two human patients. The team also presents data from a series of different animal models including successful recordings from rat barrel cortex in both acute and chronic settings; recording of the somatosensory cortex in an anesthetized pig; and recordings in non-human primates at different depths inside the brain.
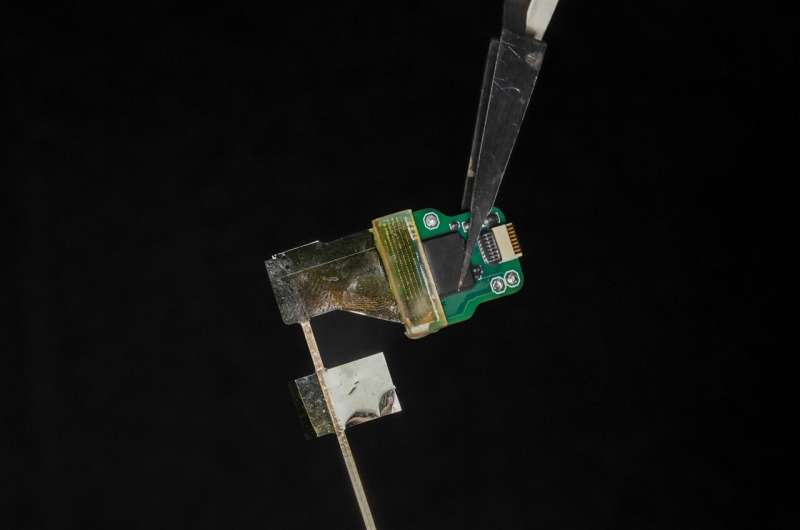
The data on the successful functioning of the device in humans were collected, with all proper approvals and consent, during already scheduled tumor-removal surgeries. During an unrelated pause in the surgery, clinicians inserted the new depth probes into brain tissue that was about to be removed.
“In a true test of the translational feasibility of the µSEEG,” the authors write in the paper, referring to the technical term for their device, “we acutely implanted short 64 channel µSEEG electrodes in the middle temporal gyrus in two separate human patient participants undergoing temporal lobe resection for clinical reasons. With each participant, we inserted a single 64-channel short µSEEG device into tissue, which the clinical team determined would be resected.” The recordings lasted 10 minutes and were able to record ongoing spontaneous activity.
Dr. Keundong Lee, first author and Postdoctoral Fellow at IEBL, UC San Diego said, “It has been a long journey since 2015 to develop a robust, human-grade depth electrode that can be used in clinical practice. Finally, we have discovered an innovative manufacturing technique to create the µSEEG probe, which can assist with high resolution and minimally invasive diagnosis of epilepsy, and potentially treatment for epilepsy and other indications, in the future.”
“Beyond epilepsy, continuous monitoring of brain activity at such high resolution could allow us to find biomarkers for other conditions, including perhaps treatment-resistant depression.”
Dr. Angelique Paulk, Instructor in Neurology at Massachusetts General Research Institute and Harvard Medical School said, “Our lab has worked with the Dayeh lab for almost a decade to bring this innovative technology to fruition. Around 2018, we tested the laminar version of the UC San Diego microSEEG in two patients at MGH.”
“Through iterative feedback that we and Drs. Sharona Ben-Haim, Ahmed Raslan, Mark Richardson, and Ziv Williams provided to inform probe fabrication, we are now happy with the end result that we feel is much closer to clinical use. We were excited to test the longer version in non-human primates here at MGH and to record the activity of single neurons with these devices.”
Dr. Sharona Ben-Haim, MD, Associate Professor of Neurological Surgery, UC San Diego School of Medicine and Surgical Director of Epilepsy, UC San Diego Health added, “This new electrode technology is exciting for a large variety of reasons, including its capacity for recording at unprecedented resolution. The future ability of this system to record wirelessly from the brain of epilepsy patients undergoing intracranial EEG evaluation has the potential to dramatically change our current clinical practice.”
“Currently, patients who undergo this type of evaluation remain in the hospital for the duration of the study, where we try to capture where their unique seizures originate during a period of time that typically lasts from 7–21 days. During this time patients are tethered to their hospital beds by the wired cords from the current clinical electrode system.”
“This new technology has the capacity to potentially allow us to send these patients home, freeing them from a long hospital stay, and potentially allowing us to record for longer periods of time and obtain more robust information to help us ultimately treat their seizures with more precision and resolution than previously possible.”
Features of the UC San Diego micro-stereo-eletro-encephalography (µSEEG)
- The probes can be up to 10 cm in length, allowing for access to structures deep within the brain.
- The probes are incredibly thin: just 15 micron thick, or one-fifth the width of a human hair, and 1.2 millimeters wide
- When inserted into brain tissue, the probe lined with sensors has a thickness that is smaller than technologies currently in clinical use. This smaller thickness means less brain tissue is damaged when the probe is inserted.
- Brain-signal recording electrodes can be placed 60 micrometers apart, which is far closer to each other than technologies currently in clinical use.
- Probes with up to 128 brain-signal-recording channels (electrodes) were demonstrated, compared to eight to 16 recording channels in today’s broadly used clinical depth electrodes.
- The small size of the electrodes allows for extremely localized brain-signal recording, as precise as the signal coming from the individual activity of one or two neurons. They can also record local field potentials, which is aggregate activity of many neurons within a brain region.
- The electrode sensors are able to record precise areas of the brain over both short and long time periods.
- The electrodes work well: they record brain activity triggered by stimulating a body part, and they record the brain dynamics known to occur during anesthesia.
- The system allowed for simultaneous recording of the cortex of the brain and signals from individual neurons deep within the brain. The researchers were able to correlate the general brain activity to what was happening at the single-neuron level.
- The system allows monitoring the dynamics of brain activity instantaneously, allowing visualization of the propagation of the activity across cortical layers with precision with time.
- Cost-effective, scalable manufacturing of the new system is in direct contrast to the expensive and time-consuming manual assembly required for the systems currently in clinical use. All other known experimental depth electrodes require some amount of manual assembly as well.
More information: Keundong Lee et al, Flexible, scalable, high channel count stereo-electrode for recording in the human brain, Nature Communications (2024). DOI: 10.1038/s41467-023-43727-9 , doi.org/10.1038/s41467-023-43727-9
News
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]