One-dimensional (1D) nanostructures exhibit distinct properties that vary from those of bulk materials. They provide significant benefits in designing next-generation batteries due to facile electronic and ionic transport and strong tolerance to stress changes. Thus, contributing to the high performance of energy storage systems.
A review article published in Advanced Functional Materials systematically reviewed the latest research on rechargeable battery-based 1D nanostructures. This review highlighted a few important 1D nanostructuring methods and summarized in situ 1D nanostructure-based structural characterizations that facilitate atomic-scale monitoring of the structural evolution dynamics and reaction kinetics of electrode materials.
The stabilization of metal anodes and 1D nanostructuring in solid-state electrolytes have also been highlighted because they are not only vital for prevailing battery research trends but are also rarely covered in earlier studies.
1D Nanostructures in Batteries
Low-dimensional nanomaterials have unique properties that are not observed in macroscale materials and have applications in rechargeable batteries. Electrode materials with 1D nanostructures facilitate fast ion or electron transport, along with large contact areas between the electrode and electrolyte.
Incorporating 1D nanostructures with hierarchical, interfacial, or tubular porous geometries further accelerates electrochemical reactions by providing several active sites, short ion diffusion lengths, and strain relaxation.
The high aspect ratio of 1D nanostructures enables self-integration, which promotes the development of three-dimensional (3D) functional structures for additional battery components. However, such self-integration is unachievable through zero-dimensional (0D) or two-dimensional (2D) nanostructures owing to their low aspect ratio.
1D nanostructures stabilized metal anodes in rechargeable batteries by rendering lithiophilic 3D interlinked nanofiber-based scaffolds, which facilitate uniform metal deposition, enhance stability during stripping and metal plating, and provide adequate channels for ion transportation.
Additionally, 1D nanostructures offer novel experimental techniques and tools for the practical determination of the physicochemical characteristics of electrode materials, providing guidelines for developing high-performance batteries.
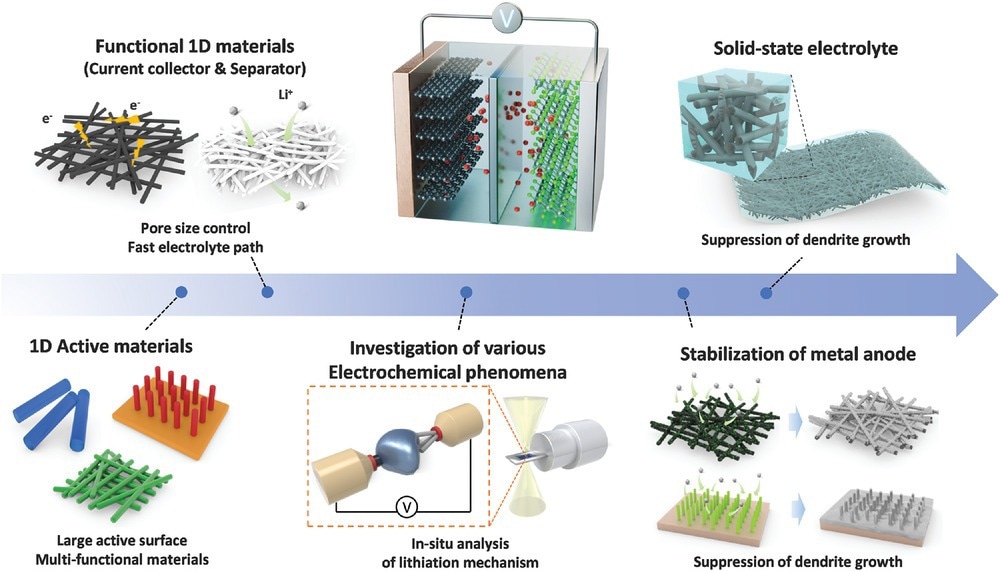
Schematic illustration on the employment of 1D nanostructures for multifunctional applications, ranging from active materials to solid-state electrolyte. © Cheong, J. Y., Cho, S.H., Lee, J., Jung, J.W., Kim, C., Kim, I.D. (2022).
Synthetic Methodologies Towards 1D Nanostructures and Their Limitations
Among the methodologies for synthesizing 1D nanostructures, solvothermal and hydrothermal synthetic strategies face several challenges, including structural predictability and control, which remain elusive in compositionally complex systems obtained via these synthetic routes. Hence, it is challenging to synthesize amorphous structures using solvothermal and hydrothermal methods.
Moreover, the formation and evolution mechanisms of various inorganic semiconducting nanostructures have been limited to hypothetical interpretations. Thus, integrating in situ transmission electron microscopy (TEM) or X-ray diffraction (XRD) into an autoclave enables the observation of reaction dynamics during crystal formation.
Chemical vapor deposition (CVD) is more favorable than the solvothermal or hydrothermal synthesis of 1D nanostructures because it allows precise or controlled synthesis. Moreover, their low production efficiency can be fixed by increasing the heating zones of the device to produce sufficient material. Although most CVD processes occur at high temperatures, synthesizing 1D nanostructures via plasma-enhanced CVD enables the process at a lower temperature.
On the other hand, the key advantages of the electrodeposition approach are low-temperature synthesis and applicability to diverse metal substrates. Nevertheless, this method is limited by the difficulty in uniformly controlling the morphology. In addition, it is challenging to employ an insulating substrate for electrodeposition using a template or a template-free method.
Although the sol-gel process is efficient, it requires a high-quality gel precursor and additional post-reaction steps to remove by-products. Electrospinning selectively facilitates the production of continuous 1D nanofibers. However, the insolubility of polymer precursors restricts their adaptability.
Alternatively, 3D printing and lithography techniques are the latest methods for preparing highly porous micro-sized lattice structures. However, these methods are limited by a lack of reliable processes for obtaining sufficient yields, a lack of suitable materials, and multistep processes in lithography techniques. Thus, by overcoming these limitations, 3D nano-printing and lithography techniques can achieve substantial development in terms of utilization and productivity in the design of energy devices.
Scope of 1D Nanostructures in Batteries
Novel battery systems such as spin- and quantum-phase batteries can be developed using 1D nanostructures. While conventional batteries provide a sustained voltage bias that can power electronic circuits by storing chemical energy, a phase-coherence-based quantum phase battery offers a quantum circuit wave function with persistent phase bias.
Owing to the unique characteristics of quantum mechanics, researchers have anticipated that batteries in the quantum phase would experience faster charging. Using 1D nanostructures has also proven the viability of rechargeable spin batteries. By applying a strong magnetic field to nanomagnets in a magnetic tunnel junction, spin batteries may store energy in magnets rather than through chemical processes.
Thus, 1D nanostructures provide enormous potential for developing new batteries and serve as a foundation for studying electrochemical dynamics using in situ or operando characterization techniques.
Conclusion
In summary, 1D nanostructures have substantially contributed to advances in battery science. The short pathways of ion or electron transport, large surface areas, and the ability for effective strain relaxation of 1D nanostructures offer excellent electrochemical performances that are not found in bulk materials.
Furthermore, constructing a nanoscale probe with a single 1D nanostructure helps investigate the electrochemical dynamics and fundamental mechanism with high resolution. This will contribute to the development of future batteries.
In addition to electrochemical analysis, integrating artificial intelligence (AI) technology is expected to contribute to significant advancements in 1D nanostructuring for real-world battery applications.
News
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]