Despite the end of the pandemic, COVID-19 continues to pose a serious health threat. Most individuals have established robust immune protection and do not develop severe disease but the infection can still lead to marked and sometimes long-lasting disease symptoms.
The researchers discovered that the pirola variant, in contrast the all previously circulating omicron variants, enters lung cells with high efficiency and uses the cellular enzyme TMPRss2 for entry, thereby exhibiting surprising parallels to variants alpha, beta, gamma and delta that circulated during the first years of the pandemic. The improved entry into lung cells might indicate that the virus is more aggressive but production of new, infectious viral particles in infected cells was reduced, which may limit spread and pathogenic potential.
The researchers report in the journal Cell that the pirola variant is resistant against all therapeutic antibodies and efficiently evades antibody responses in vaccinated individuals with and without breakthrough infection. However, the virus was appreciably inhibited by antibodies elicited by the new, XBB.1.5-adpated mRNA vaccine.
In summary, the results show that four years after the start of the pandemic the virus is still capable of profound changes and can reacquire properties that may promote the development of severe disease.
The spread of SARS-CoV-2 is associated with the constant emergence of new viral variants. These variants have acquired mutations in the spike protein, which allow evasion of neutralizing antibodies in vaccinated and convalescent individuals. The emergence of viral variants started with the alpha variant followed by the beta, gamma and delta variants.
At the end of the year 2021 the omicron variant became globally dominant, which, based on genome sequence, differed markedly from previously circulating variants. However, the virus had to pay a price for this massive change. Thus, the omicron variant evades neutralizing antibodies and is transmitted with high efficiency but has lost the ability to efficiently use a host cell enzyme, the protease TMPRSS2, for lung cell entry. As a consequence, the omicron variant induces pneumonia less frequently.
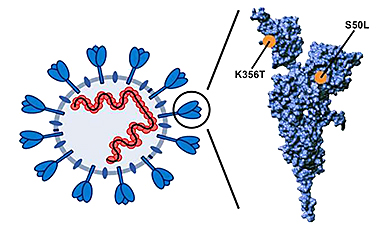
Pirola: A quantum leap in SARS-CoV-2 evolution
Descendants of the omicron variant dominated globally until the end of the year 2023. New variants frequently differed only by few mutations from their predecessors and there was evidence that viruses circulating in 2023 had only limited options to evade antibody pressure in the human population. Therefore, the discovery of a new SARS-CoV-2 omicron subvariant, pirola (BA.2.86), which, based on genome sequence, strongly differed from other circulating viruses drew a lot of attention.
The pirola variant, analogous to the omicron variant, likely arose in immunocompromised patients and presents a quantum leap in SARS-CoV-2 evolution. The spike protein of the pirola variant harbors more than 30 mutations relative to its precursor variant, BA.2, and it is largely unknown how these mutations affect the biological properties of the virus.
A team of researchers from the German Primate Center (DPZ) led by Markus Hoffmann and Stefan Pöhlmann addressed this question jointly with the research groups of Christian Drosten (Charité, Berlin), Georg Behrens (Hannover Medical School), Luka Cicin-Sain (Helmholtz Center for Infection Research, Braunschweig) and Hans-Martin Jäck (Friedrich-Alexander-University Erlangen Nuremberg).
Pirola can infect lung cells more efficiently
The researchers discovered that the pirola variant, in contrast to all previously circulating omicron subvariants, enters lung cells with high efficiency and in a TMPRSS2-dependent manner. Further, they could demonstrate that mutations S50L and K356T in the spike protein of the pirola variant are important for the highly efficient lung cell entry.
“It is noteworthy that two years after the global dominance of the omicron variant, which fails to robustly enter lung cells, now a quite different virus is spreading and that this virus is able to again enter lung cells with high efficiency. If the augmented lung cell entry translates into more severe disease upon infection with the pirola variant remains to be investigated in animal models,” says Pöhlmann, head of the Infection Biology Unity of the German Primate Center.
Pirola replicates less well than its predecessors
SARS-CoV-2 infected cells produce new virus particles many of which, but not all, are able to infect new cells. The researchers provided evidence that cells infected by the pirola variant are less well able than cells infected with previous variants to produce intact viral particles. “The relatively inefficient production of infectious particles by cells infected with the pirola variant was surprising,” says Hoffmann, the lead contact of the study.
“It will be interesting to analyze which mechanism is responsible. Maybe the infected cells produce defective interfering particles, which regulate spread of the pirola variant and contribute to antibody evasion.”
Therapeutic antibodies are ineffective against pirola
Recombinantly produced neutralizing antibodies were successfully used for COVID-19 prophylaxis and therapy. However, due to the emergence of viral variants with mutations in the antibody binding sites most of those antibodies are not active against currently circulating variants. The present study shows that the pirola variant is no exception—none of the tested antibodies was able to neutralize the virus.
“These results show that the development of new, broad spectrum antibodies is an important task,” says Hoffmann.
New, adapted vaccine protects against pirola
The pirola variant was also able to evade antibodies induced by vaccination or infection but with less efficiency than the contemporaneously circulating Eris variant (EG.5.1). However, antibodies induced by vaccination with the new XBB.1.5-adapted vaccine were able to appreciably inhibit both the pirola and the Eris variant.
“These results suggest that the XBB.1.5-adpated vaccine might induce a robust, although likely short-lived, protection against infection with the pirola variant,” says Hoffmann.
“In this context it is interesting that subvariants of pirola are currently globally on the rise that harbor an additional mutation in the spike protein, which may increase antibody evasion. The virus is in the process of optimizing itself and the consequences of this optimization should be studied,” adds Lu Zhang, first author of the study.
More information: Lu Zhang et al, SARS-CoV-2 BA.2.86 enters lung cells and evades neutralizing antibodies with high efficiency, Cell (2024). DOI: 10.1016/j.cell.2023.12.025
News
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]