The delta variant within the SARS-CoV-2 (COVID-19) virus continued to cause devastation with high infection rates and transmissibility and this illustrated the lack of efficacy by the SARS-CoV-2 vaccines. However, novel research published in the journal, ACS Omega has reported the use of human host defense peptide-conjugated graphene quantum dots for the prevention of virus entry into host cells.
The severe acute respiratory coronavirus 2 (SARS-CoV-2) caused a global health crisis, which the World Health Organization (WHO) named to be a pandemic in March 2019. This virus was responsible for over 5.4 million deaths worldwide and has become a very intriguing research point for researchers studying its many emerging variants.
Research on this evolving virus mainly focuses on the spike protein (S1) which contains the receptor-binding domain (RBD) and the binding of this to the angiotensin converting enzyme 2 (ACE2) receptor within epithelial cells enables the virus to enter host cells in humans. This research resulted in the spike protein becoming a target for vaccines that aimed to produce neutralizing antibodies against the S-RBD.
While this concept seemed useful, recent reports have suggested the mutations on the SARS-CoV-2 virus and specifically in the S-RBD, can cause a decline in the level of neutralizing antibodies against the delta (B.1.617.2) variant that may have been produced during a previous infection or from immunization via a vaccine.
With these reports coming to light, researchers have strategized a novel and innovative solution for blocking the interaction between the spike protein and the ACE2 receptor to prevent infections from mutating variants.
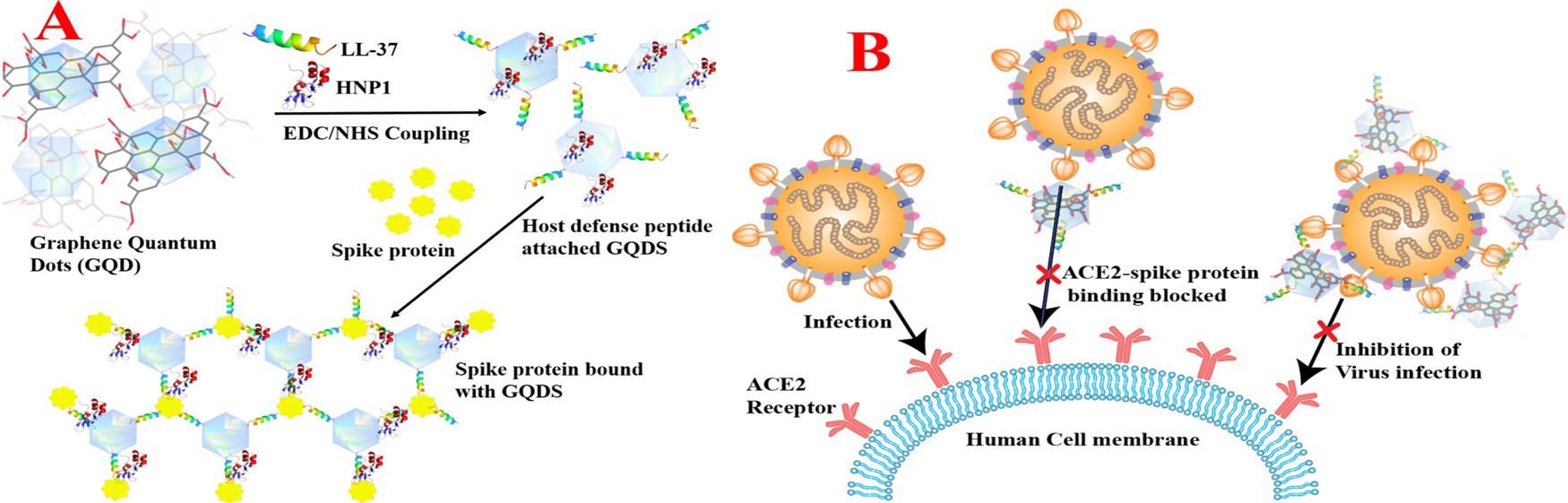
Figure 1. (A) Scheme showing the design of HNP1 and LL-37 human host defense peptide-conjugated GQDs and binding of HNP1 and LL-37 peptide-conjugated GQDs in the presence of the SARS-CoV-2 delta variant (B.1.617.2) spike protein RBD. (B) Scheme showing the blocking of the S-RBD interaction with ACE2 on a human cell membrane and preventing the SARS-CoV-2 virus entry. © Pramanik, A., et al. (2022)
Quantum Dot Solution to COVID-19
This novel research is premised on the fact that approximately 42% of the infected population are asymptomatic, which can suggest that the COVID-19 infection can be effectively controlled by the innate immune system.
This system is the first defense against pathogens coming into the body such as viruses and bacteria; this activates an immune response to destroy the pathogen while the adaptive immune response is modulated which causes immune cells to multiply and fight against the infection and ultimately, result in recovery.
Critical components of the innate immune system consist of peptides such as α-Defensin human neutrophil peptides (HNP1, HNP2, HNP3, and HNP4) and human β-defensins (HBD1, HBD2, and HBD3) as well as LL-37 (leucine-leucine-37) cathelicidin family peptides.
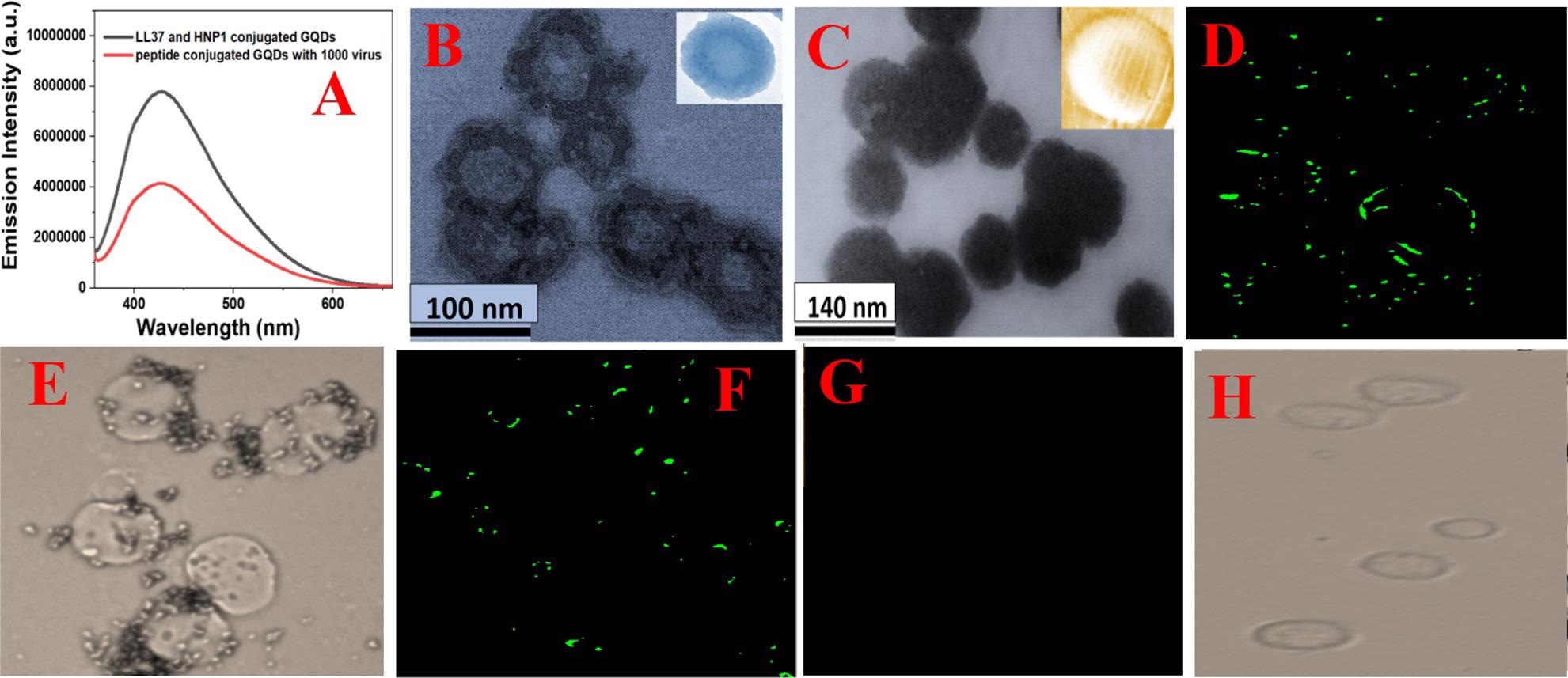
Figure 2. (A) Fluorescence spectra from HNP1 and LL-37 peptide-conjugated GQDs in the presence and absence of GFP-tagged Baculovirus pseudotyped with a SARS-CoV-2 delta variant (B.1.617.2) spike protein. (B) TEM image of Baculovirus pseudotyped after they are treated with HNP1 human host defense peptide-attached GQDs for 30 min. (C) TEM image of Baculovirus pseudotyped after they are treated with HNP1 and LL-37 human host defense peptide-attached GQDs for 30 min. (D–H) Inhibition of SARS-CoV-2 spike protein binding to the surface of HEK-293T cells expressing ACE2. The green fluorescence is due to the presence of GFP-tagged Baculovirus pseudotyped with a SARS-CoV-2 delta variant (B.1.617.2) spike protein on the surface of HEK-293T cells expressing ACE2. (D) Fluorescence image of HEK-293T cells in the presence of GFP-tagged pseudotyped delta virus without GQDs. (E) Bright-field image of HEK-293T cells in the presence of GFP-tagged Baculovirus pseudotyped without GQDs. (F) Fluorescence image of HEK-293T cells in the presence of GFP-tagged virus bound with LL-37 human host defense peptide-attached GQDs. (G) Fluorescence image of HEK-293T cells in the presence of GFP-tagged virus bound with LL-37 & HNP1 human host defense peptide-attached GQDs. (H) Bright-field image of HEK-293T cells in the presence of GFP-tagged virus bound with LL-37 & HNP1 human host defense peptide-attached GQDs. © Pramanik, A., et al. (2022)
Defensins and cathelicidin peptides hold an important function in viral inhibition through binding and destabilization.
Researchers of this study aimed to block the delta variant and the subsequent infection from the SARS-CoV-2 virus through the use of an innovative design of HNP1 and LL-37 peptide-conjugated graphene quantum dots (GQDs). This novel development has the ability to bind to the delta variant S-RBD and block the binding to the ACE2 receptor in host cells, preventing the virus from entering the host cell, and therefore prevent COVID-19 infection.
The development of graphene quantum dots is a novel innovation that comprises a graphene lattice as well as graphene sheets that exhibit size-dependent luminescence properties due to quantum confinement and edge effects. These GQDs consist of surface groups including, carboxy, epoxy, and hydroxyl which illustrate high water solubility, high surface area as well as high photostability.
The unique optical properties of GQDs allow this candidate to be highly useful in applications such as bioimaging and biosensing; however, it can also be used innovatively to monitor the status of the delta variant of the SARS-CoV-2 virus.
The bioconjugated GQD fluorescence in this research study has been used to monitor the spike RBD and ACE2 receptor interaction to determine the effective binding affinity. Additionally, the functional groups on the GQDs have also been used to inactivate the virus through decomposing the lipid membrane of the virus and removing the spike proteins attached to the lipid membrane.
Translational Significance
This research illustrates the double usage of this quantum dot strategy which would first be used to monitor the delta variant and compete for the S-RBD-ACE2 interaction to prevent this critical attachment, as well as attempt to remove the spike proteins from the lipid membrane to also prevent this interaction.
The binding affinity for the delta variant in comparison to the alpha, beta, and gamma variant spike-RBD was shown to be higher using this innovative strategy. This can be useful as this advancement would enable the complete inhibition of the delta variant from the host cell.
This research would enhance the defense against the coronavirus and its emerging variants, with the possibility of modification for other emerging variants of concern. Additionally, this could also be translated for use against other viruses in order to increase protection against pathogens.
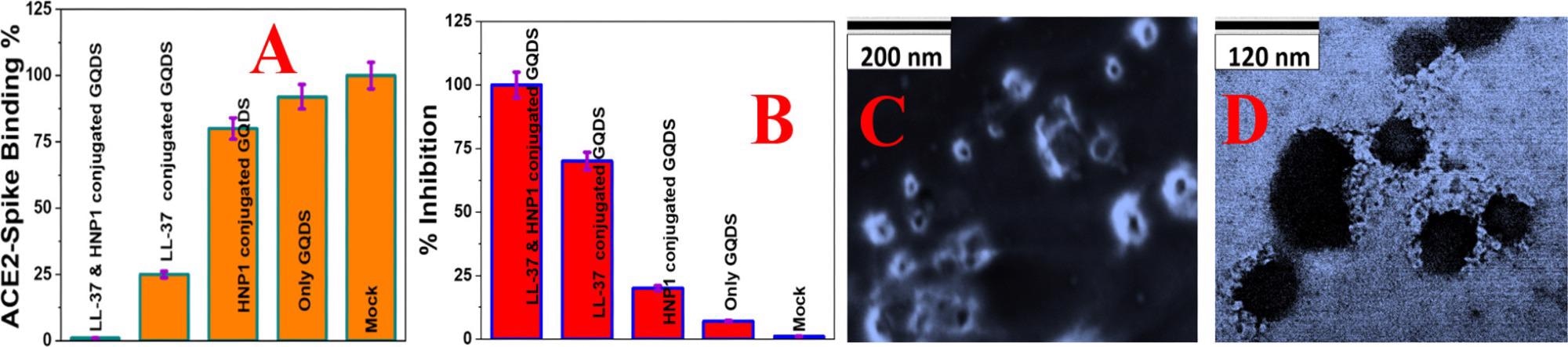
Figure 3. (A) Interaction of Baculovirus pseudotyped with a SARS-CoV-2 delta variant (B.1.617.2) spike protein and ACE2 on HEK-293T cells, measured using fluorescence imaging. (B) Inhibition efficiency of Baculovirus pseudotyped with the delta variant spike protein in infected HEK293T cells in the presence of buffer (Mock), GQDs (30 μg/mL), HNP1 (4 μg/mL)-attached GQDs (30 μg/mL), LL-37 (4 μg/mL)-attached GQDs (30 μg/mL), and LL-37 (4 μg/mL) and HNP1 (4 μg/mL)-attached GQDs (30 μg/mL). (C) SEM image of Baculovirus pseudotyped with a SARS-CoV-2 delta variant (B.1.617.2) spike protein when they are treated with peptide-attached GQDs for 6 h. (D) TEM image of Baculovirus pseudotyped with a SARS-CoV-2 delta variant (B.1.617.2) spike protein when they are treated with peptide-attached GQDs for 12 h. © Pramanik, A., et al. (2022)
News
Repurposed drugs could calm the immune system’s response to nanomedicine
An international study led by researchers at the University of Colorado Anschutz Medical Campus has identified a promising strategy to enhance the safety of nanomedicines, advanced therapies often used in cancer and vaccine treatments, [...]
Nano-Enhanced Hydrogel Strategies for Cartilage Repair
A recent article in Engineering describes the development of a protein-based nanocomposite hydrogel designed to deliver two therapeutic agents—dexamethasone (Dex) and kartogenin (KGN)—to support cartilage repair. The hydrogel is engineered to modulate immune responses and promote [...]
New Cancer Drug Blocks Tumors Without Debilitating Side Effects
A new drug targets RAS-PI3Kα pathways without harmful side effects. It was developed using high-performance computing and AI. A new cancer drug candidate, developed through a collaboration between Lawrence Livermore National Laboratory (LLNL), BridgeBio Oncology [...]
Scientists Are Pretty Close to Replicating the First Thing That Ever Lived
For 400 million years, a leading hypothesis claims, Earth was an “RNA World,” meaning that life must’ve first replicated from RNA before the arrival of proteins and DNA. Unfortunately, scientists have failed to find [...]
Why ‘Peniaphobia’ Is Exploding Among Young People (And Why We Should Be Concerned)
An insidious illness is taking hold among a growing proportion of young people. Little known to the general public, peniaphobia—the fear of becoming poor—is gaining ground among teens and young adults. Discover the causes [...]
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]