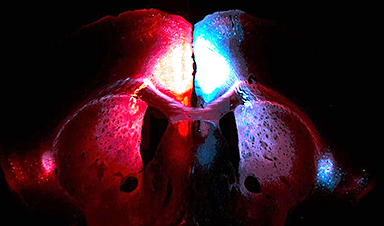
Researchers used advanced microscopy to uncover important protein structures.
For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of Cincinnati’s Center for Advanced Structural Biology. This breakthrough could lead to the development of more effective therapeutics.
The research, conducted by a team of structural biologists at UC, was published in the Proceedings of the National Academy of Sciences (PNAS).
This is the first publication from the Seegar Lab at UC. Tom Seegar, PhD, Ohio Eminent Scholar and assistant professor in the Department of Molecular and Cellular Biosciences in the College of Medicine, is the corresponding author.
The study’s first authors are Joe Maciag, PhD, a research scientist in the Seegar Lab, and Conner Slone, a graduate student assistant in the same lab.
For the first time, they are visualizing the physical, atomic structure of two protein complexes, observing how they interact, how their functions change, and how this process drives inflammatory signaling.
“If you can see something, you can figure out how it works,” said Seegar. “We are figuring out what this enzyme looks like and how it’s regulated.”
What they saw
The Seegar Lab used advanced cryogenic electron microscopy to reveal the structure of the ADAM17 enzyme bound to its regulator protein, iRhom2, and identified key features essential for its activity. They also pinpointed the critical interaction within the ADAM17-iRhom2 complex that governs how protein substrates are processed.
Scientists have long known that ADAM17 is a vital enzyme found in all cells, playing a key role in development and immune system regulation. It becomes dysregulated in chronic inflammatory diseases and has been linked to conditions such as rheumatoid arthritis, cancer, and Covid-19.

Researchers are now studying how ADAM17 communicates with other proteins involved in immune response and tissue repair. “We know in some cancers and rheumatoid arthritis, way too much signaling is occurring,” said Maciag. “But some treatments create too many side effects, worse than the disease itself.”
Maciag explained that the team is exploring ways to target iRhom2 for more precise therapeutic approaches. They have already identified structural features inside the cell, called the “re-entry loop” of iRhom2, that transmit information from the cell’s interior to its exterior. These structures are essential for ADAM17 to function outside the cell and had not been well understood until now.
Seegar emphasized the human impact. “This work provides a foundation for designing therapies targeting ADAM17-related diseases, offering new strategies to address critical health conditions,” he said.
New research core facility
The ADAM17-iRhom2 Complex contains the first protein structures coming out of UC’s Center for Advanced Structural Biology, established in 2022.
“We are indebted to UC,” said Seegar. “Our work wouldn’t be possible without this research core facility.”
The cryogenic electron microscopy technology being used has transformed structural biology. The center’s focal point is its transmission electron microscope (TEM), which is ideally suited for screening cryo-EM samples and allows researchers to see complex proteins without leaving UC’s campus.
“It’s a privilege to have this microscope in house, and it’s exciting to use it to solve these structures,” said Maciag. “It’s important to our field of cellular biology and will help drive research forward and how we approach our understanding of inflammation in known disease states.”
The Seegar Lab used UC’s Advanced Research Computing Center (ARC) for data processing, allowing them to keep their data, used to create a 3D model of the ADAM17-iRhom2 Complex, on campus as well.
Moving forward, Seegar’s lab plans to research iRhom2 more closely.
“These adapter proteins are not well understood,” said Slone. “Our research will be in understanding them and will be driven by specificity. Ideally, controlling these will allow researchers to control disease states.”
Reference: “Structural insights into the activation and inhibition of the ADAM17–iRhom2 complex” by Joseph J. Maciag, Conner E. Slone, Hala F. Alnajjar, Maria F. Rich, Bryce Guion, Igal Ifergan, Carl P. Blobel and Tom C. M. Seegar, 13 June 2025, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2500732122
The study was funded by the National Institute of General Medicine Sciences and with support from a University of Cincinnati Research Innovation/Pilot Grant.
News
Scientists Rewire Natural Killer Cells To Attack Cancer Faster and Harder
Researchers tested new CAR designs in NK-92 cells and found the modified cells killed tumor cells more effectively, showing stronger anti-cancer activity. Researchers at the Ribeirão Preto Blood Center and the Center for Cell-Based [...]
New “Cellular” Target Could Transform How We Treat Alzheimer’s Disease
A new study from researchers highlights an unexpected player in Alzheimer’s disease: aging astrocytes. Senescent astrocytes have been identified as a major contributor to Alzheimer’s progression. The cells lose protective functions and fuel inflammation, particularly in [...]
Treating a Common Dental Infection… Effects That Extend Far Beyond the Mouth
Successful root canal treatment may help lower inflammation associated with heart disease and improve blood sugar and cholesterol levels. Treating an infected tooth with a successful root canal procedure may do more than relieve [...]
Microplastics found in prostate tumors in small study
In a new study, researchers found microplastics deep inside prostate cancer tumors, raising more questions about the role the ubiquitous pollutants play in public health. The findings — which come from a small study of 10 [...]
All blue-eyed people have this one thing in common
All Blue-Eyed People Have This One Thing In Common Blue Eyes Aren’t Random—Research Traces Them Back to One Prehistoric Human It sounds like a myth at first — something you’d hear in a folklore [...]
Scientists reveal how exercise protects the brain from Alzheimer’s
Researchers at UC San Francisco have identified a biological process that may explain why exercise sharpens thinking and memory. Their findings suggest that physical activity strengthens the brain's built in defense system, helping protect [...]
NanoMedical Brain/Cloud Interface – Explorations and Implications. A new book from Frank Boehm
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
Deadly Pancreatic Cancer Found To “Wire Itself” Into the Body’s Nerves
A newly discovered link between pancreatic cancer and neural signaling reveals a promising drug target that slows tumor growth by blocking glutamate uptake. Pancreatic cancer is among the most deadly cancers, and scientists are [...]
This Simple Brain Exercise May Protect Against Dementia for 20 Years
A long-running study following thousands of older adults suggests that a relatively brief period of targeted brain training may have effects that last decades. Starting in the late 1990s, close to 3,000 older adults [...]
Scientists Crack a 50-Year Tissue Mystery With Major Cancer Implications
Researchers have resolved a 50-year-old scientific mystery by identifying the molecular mechanism that allows tissues to regenerate after severe damage. The discovery could help guide future treatments aimed at reducing the risk of cancer [...]
This New Blood Test Can Detect Cancer Before Tumors Appear
A new CRISPR-powered light sensor can detect the faintest whispers of cancer in a single drop of blood. Scientists have created an advanced light-based sensor capable of identifying extremely small amounts of cancer biomarkers [...]
Blindness Breakthrough? This Snail Regrows Eyes in 30 Days
A snail that regrows its eyes may hold the genetic clues to restoring human sight. Human eyes are intricate organs that cannot regrow once damaged. Surprisingly, they share key structural features with the eyes [...]
This Is Why the Same Virus Hits People So Differently
Scientists have mapped how genetics and life experiences leave lasting epigenetic marks on immune cells. The discovery helps explain why people respond so differently to the same infections and could lead to more personalized [...]
Rejuvenating neurons restores learning and memory in mice
EPFL scientists report that briefly switching on three “reprogramming” genes in a small set of memory-trace neurons restored memory in aged mice and in mouse models of Alzheimer’s disease to level of healthy young [...]
New book from Nanoappsmedical Inc. – Global Health Care Equivalency
A new book by Frank Boehm, NanoappsMedical Inc. Founder. This groundbreaking volume explores the vision of a Global Health Care Equivalency (GHCE) system powered by artificial intelligence and quantum computing technologies, operating on secure [...]
New Molecule Blocks Deadliest Brain Cancer at Its Genetic Root
Researchers have identified a molecule that disrupts a critical gene in glioblastoma. Scientists at the UVA Comprehensive Cancer Center say they have found a small molecule that can shut down a gene tied to glioblastoma, a [...]