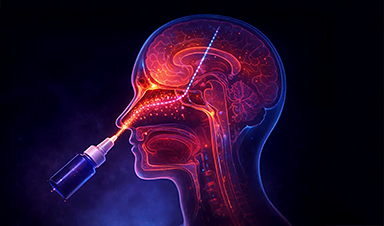
Scientists have developed platelet-inspired nanoparticles that deliver anti-inflammatory drugs directly to brain-computer interface implants, doubling their effectiveness.
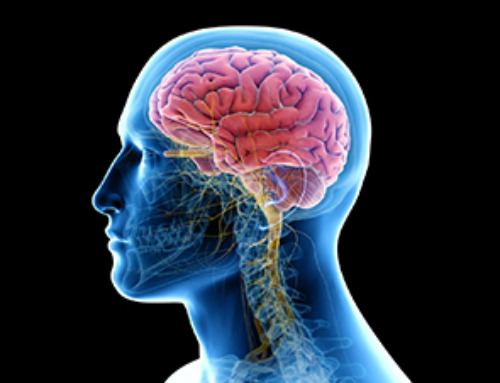
Scientists have found a way to improve the performance of brain-computer interface (BCI) electrodes by delivering anti-inflammatory drugs directly to the site of implantation. The study, led by researchers at Case Western Reserve University (CWRU) in collaboration with Haima Therapeutics, has the potential to treat a wide range of inflammatory diseases and improve devices that let people control prosthetic limbs with their thoughts.
Nanoparticles as a Trojan horse
The team used a novel ‘platelet-inspired nanoparticle’ to transport an anti-inflammatory drug directly to the brain where BCI electrodes are implanted, which doubled the effectiveness of the electrodes.
“When we implant devices in the brain, it disrupts the blood-brain barrier, and we knew platelets would be showing up to seal the breach,” said neural engineer Andrew Shoffstall, the Nord Distinguished Associate Professor of Biomedical Engineering in the Case School of Engineering and Case Western Reserve School of Medicine. “We used these nanoparticles that act like platelets as a Trojan horse to target the site where we’re putting the device.”
How brain-computer interfaces work
BCI technology allows people with spinal cord injuries or prosthetic limbs to operate devices or limbs by thinking about them. Electrodes implanted in the brain sense neuronal activity and translate it into commands. However, inflammation around the implants can interfere with signals, limiting the lifespan and effectiveness of the electrodes.
By delivering the drug directly using nanoparticles, the team improved electrode performance.
“The brain recognises the implant as a foreign object – like a splinter – and it responds with inflammation to try to isolate and neutralise it,” Shoffstall explained.
By delivering the drug directly using nanoparticles, the team improved electrode performance, whereas administering the same drug systemically actually worsened results. The team now plans to move the research towards translation, starting with safety studies.
Synthetic platelets enable precision delivery
The approach relies on synthetic platelet technology developed by Anirban Sen Gupta, Wallace R. Persons professor of biomedical engineering, who patented it and licensed it to Haima Therapeutics, a biotech start-up he co-founded with CWRU alumna and COO Christa Pawlowski.
The technology, known as SynthoPlate, could have wider applications, including controlling life-threatening bleeding and targeted drug delivery.
Future clinical applications for different diseases
“Because the particle itself is a platform, you can load it with any drug, as long as you pick a disease or pathology where platelets can accumulate,” Sen Gupta said. “Really, it has potential for treating any disease that involves vascular injury and inflammation, from stroke and heart attack to autoimmune disorders like rheumatoid arthritis or infectious diseases like sepsis.”
Because the particle itself is a platform, you can load it with any drug.
Haima Therapeutics plans to begin human clinical trials of the platelet-inspired nanoparticles in 2027 and recently secured two Small Business Innovation Research Grants from the Defense Advanced Research Projects Agency (DARPA) to advance the technology towards clinical translation.
News
Scientists Discover a New “Cleanup Hub” Inside the Human Brain
A newly identified lymphatic drainage pathway along the middle meningeal artery reveals how the human brain clears waste. How does the brain clear away waste? This task is handled by the brain’s lymphatic drainage [...]
New Drug Slashes Dangerous Blood Fats by Nearly 40% in First Human Trial
Scientists have found a way to fine-tune a central fat-control pathway in the liver, reducing harmful blood triglycerides while preserving beneficial cholesterol functions. When we eat, the body turns surplus calories into molecules called [...]
A Simple Brain Scan May Help Restore Movement After Paralysis
A brain cap and smart algorithms may one day help paralyzed patients turn thought into movement—no surgery required. People with spinal cord injuries often experience partial or complete loss of movement in their arms [...]
Plant Discovery Could Transform How Medicines Are Made
Scientists have uncovered an unexpected way plants make powerful chemicals, revealing hidden biological connections that could transform how medicines are discovered and produced. Plants produce protective chemicals called alkaloids as part of their natural [...]
Scientists Develop IV Therapy That Repairs the Brain After Stroke
New nanomaterial passes the blood-brain barrier to reduce damaging inflammation after the most common form of stroke. When someone experiences a stroke, doctors must quickly restore blood flow to the brain to prevent death. [...]
Analyzing Darwin’s specimens without opening 200-year-old jars
Scientists have successfully analyzed Charles Darwin's original specimens from his HMS Beagle voyage (1831 to 1836) to the Galapagos Islands. Remarkably, the specimens have been analyzed without opening their 200-year-old preservation jars. Examining 46 [...]
Scientists discover natural ‘brake’ that could stop harmful inflammation
Researchers at University College London (UCL) have uncovered a key mechanism that helps the body switch off inflammation—a breakthrough that could lead to new treatments for chronic diseases affecting millions worldwide. Inflammation is the [...]
A Forgotten Molecule Could Revive Failing Antifungal Drugs and Save Millions of Lives
Scientists have uncovered a way to make existing antifungal drugs work again against deadly, drug-resistant fungi. Fungal infections claim millions of lives worldwide each year, and current medical treatments are failing to keep pace. [...]
Scientists Trap Thyme’s Healing Power in Tiny Capsules
A new micro-encapsulation breakthrough could turn thyme’s powerful health benefits into safer, smarter nanodoses. Thyme extract is often praised for its wide range of health benefits, giving it a reputation as a natural medicinal [...]
Scientists Develop Spray-On Powder That Instantly Seals Life-Threatening Wounds
KAIST scientists have created a fast-acting, stable powder hemostat that stops bleeding in one second and could significantly improve survival in combat and emergency medicine. Severe blood loss remains the primary cause of death from [...]
Oceans Are Struggling To Absorb Carbon As Microplastics Flood Their Waters
New research points to an unexpected way plastic pollution may be influencing Earth’s climate system. A recent study suggests that microscopic plastic pollution is reducing the ocean’s capacity to take in carbon dioxide, a [...]
Molecular Manufacturing: The Future of Nanomedicine – New book from Frank Boehm
This book explores the revolutionary potential of atomically precise manufacturing technologies to transform global healthcare, as well as practically every other sector across society. This forward-thinking volume examines how envisaged Factory@Home systems might enable the cost-effective [...]
New Book! NanoMedical Brain/Cloud Interface – Explorations and Implications
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
Global Health Care Equivalency in the Age of Nanotechnology, Nanomedicine and Artificial Intelligence
A new book by Frank Boehm, NanoappsMedical Inc. Founder. This groundbreaking volume explores the vision of a Global Health Care Equivalency (GHCE) system powered by artificial intelligence and quantum computing technologies, operating on secure [...]
Miller School Researchers Pioneer Nanovanilloid-Based Brain Cooling for Traumatic Injury
A multidisciplinary team at the University of Miami Miller School of Medicine has developed a breakthrough nanodrug platform that may prove beneficial for rapid, targeted therapeutic hypothermia after traumatic brain injury (TBI). Their work, published in ACS [...]
COVID-19 still claims more than 100,000 US lives each year
Centers for Disease Control and Prevention researchers report national estimates of 43.6 million COVID-19-associated illnesses and 101,300 deaths in the US during October 2022 to September 2023, plus 33.0 million illnesses and 100,800 deaths [...]