Scientists have found a way to fine-tune a central fat-control pathway in the liver, reducing harmful blood triglycerides while preserving beneficial cholesterol functions.
When we eat, the body turns surplus calories into molecules called "triglycerides", especially when those calories come from carbs, sugar, fats, and alcohol. Triglycerides are a type of fat or "lipid", and the body stores them in fat cells to use as fuel between meals.
However, too much of this fat can become harmful. High triglyceride levels can lead to "hypertriglyceridemia" ("excess triglycerides in the blood"), a condition tied to a much higher risk of heart disease, stroke, and pancreatitis. That is why people are widely encouraged to support healthy triglyceride levels through diet and exercise, while more severe cases may require medication.
Dialing down a receptor
Healthy blood fat levels rely on a balance between how much fat enters circulation and how quickly it is removed. The liver and intestine send fat carrying particles into the bloodstream, and enzymes help break them down so the body can clear them. If the body produces more of these fats than it can process, triglycerides accumulate and can contribute to disorders such as dyslipidemia, acute pancreatitis, and metabolic dysfunction-associated steatotic liver disease (MASLD).
A key regulator in this network is the Liver X Receptor, or LXR, a protein that controls multiple genes involved in how the body produces and manages fats.
When LXR activity increases, triglycerides and cholesterol often climb as well. Reducing LXR signaling with a drug could be useful, but there is a catch. Because LXR also supports protective cholesterol related pathways in other tissues, shutting it down throughout the body could create unwanted effects. This tradeoff has made it difficult to turn LXR into a safe treatment target.
A drug that specifically targets liver LXR
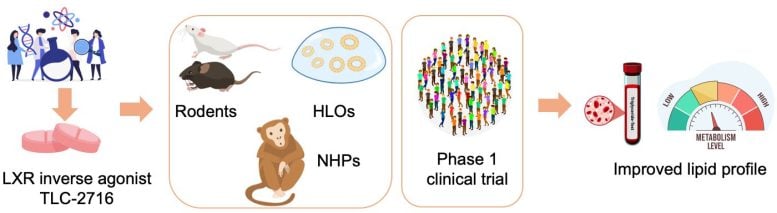
Researchers led by Johan Auwerx at EPFL and Mani Subramanian at OrsoBio have now developed an orally administered compound designed to curb LXR activity mainly in the liver and gut. The goal is to lower triglycerides while leaving the body's protective cholesterol pathways intact.
The drug, TLC-2716, is described as an "inverse agonist" for LXR. Unlike a "blocker" ("antagonist") that simply prevents activation, an "inverse agonist" pushes the receptor toward the reverse of its usual signaling.
The work, published in Nature Medicine, is the first study of this approach to be tested in humans.
Combing genetic datasets to find the right receptor variant
The scientists began by analyzing large human genetics datasets to determine which LXR variant is related to biomarkers for elevated triglycerides in the blood. The data pointed to the genetic variants within LXRα, which is highly expressed in the liver.
This was further confirmed through "Mendelian randomization", a powerful method that determines causal relationships between gene expression and outcomes. In this case, it confirmed a causal link between LXRα and metabolic disorders: higher LXRα expression can drive triglycerides upward.
The findings helped select TLC‑2716 as an effective compound to test against LXRα.
Testing the compound
The study then moved from computers into the lab. In rodent models of metabolic disease, TLC‑2716 and a related compound lowered triglycerides and cholesterol in the blood and reduced fat accumulation in the liver. Meanwhile, experiments in human liver organoids (miniature lab-grown models of diseased liver tissue), showed the same trend, with less lipid buildup and lower inflammation and fibrosis.
Next was safety. Toxicology studies in mice and non-human primates, combined with pharmacokinetic analyses, showed that TLC‑2716 largely stays in the liver and gut. This is key, as it limits exposure to other tissues where inhibiting LXR could be risky, thus addressing the main problem of developing drugs for treating metabolic diseases related to high triglycerides in the body.
The clinical trial
The lab findings set the stage for a randomized, placebo-controlled Phase 1 study in healthy adults. Participants received TLC‑2716 for 14 days, given as a single dose per day, and the trial focused first on safety and tolerability, and the authors report that the drug met these primary endpoints.
But even this short trial had clear effects: participants who received higher doses of TLC‑2716 showed notable drops in triglycerides as well as remnant cholesterol. At the highest doses of TLC‑2716 (12mg), triglycerides fell by up to 38.5%, while postprandial ("after eating") remnant cholesterol dropped by as much as 61%. This happened despite participants starting with relatively normal lipid levels and without the use of other lipid-lowering drugs.
The treatment also sped up triglyceride clearance by reducing the activity of two proteins that normally slow it down, ApoC3 and ANGPTL3. At the same time, the study did not detect reductions in blood-cell expression of ABCA1 and ABCG1, genes used here as markers linked to reverse cholesterol transport.
The trial's results show that selectively reducing LXR activity in the liver and gut by TLC‑2716 may offer a new way, complementary to other approaches, to tackle high triglycerides and related metabolic disorders. The Phase 1 data support further clinical testing in Phase 2 studies, including in people with hypertriglyceridemia and MASLD. Larger trials will be needed, but, for now, the concept has its first human proof of principle.
Reference: "An oral, liver-restricted LXR inverse agonist for dyslipidemia: preclinical development and phase 1 trial" by Xiaoxu Li, Giorgia Benegiamo, Archana Vijayakumar, Natalie Sroda, Masaki Kimura, Ryan S. Huss, Steve Weng, Eisuke Murakami, Brian J. Kirby, Giacomo V. G. von Alvensleben, Claus Kremoser, Edward J. Gane, Takanori Takebe, Robert P. Myers, G. Mani Subramanian and Johan Auwerx, 16 January 2026, Nature Medicine.
DOI: 10.1038/s41591-025-04169-6
Funding: École Polytechnique Fédérale de Lausanne (EPFL), NIH/National Institutes of Health, Japan Agency for Medical Research and Development, Japan World Premier International Research Center Initiative (WPI), OrsoBio
News
New Drug Slashes Dangerous Blood Fats by Nearly 40% in First Human Trial
Scientists have found a way to fine-tune a central fat-control pathway in the liver, reducing harmful blood triglycerides while preserving beneficial cholesterol functions. When we eat, the body turns surplus calories into molecules called [...]
A Simple Brain Scan May Help Restore Movement After Paralysis
A brain cap and smart algorithms may one day help paralyzed patients turn thought into movement—no surgery required. People with spinal cord injuries often experience partial or complete loss of movement in their arms [...]
Plant Discovery Could Transform How Medicines Are Made
Scientists have uncovered an unexpected way plants make powerful chemicals, revealing hidden biological connections that could transform how medicines are discovered and produced. Plants produce protective chemicals called alkaloids as part of their natural [...]
Scientists Develop IV Therapy That Repairs the Brain After Stroke
New nanomaterial passes the blood-brain barrier to reduce damaging inflammation after the most common form of stroke. When someone experiences a stroke, doctors must quickly restore blood flow to the brain to prevent death. [...]
Analyzing Darwin’s specimens without opening 200-year-old jars
Scientists have successfully analyzed Charles Darwin's original specimens from his HMS Beagle voyage (1831 to 1836) to the Galapagos Islands. Remarkably, the specimens have been analyzed without opening their 200-year-old preservation jars. Examining 46 [...]
Scientists discover natural ‘brake’ that could stop harmful inflammation
Researchers at University College London (UCL) have uncovered a key mechanism that helps the body switch off inflammation—a breakthrough that could lead to new treatments for chronic diseases affecting millions worldwide. Inflammation is the [...]
A Forgotten Molecule Could Revive Failing Antifungal Drugs and Save Millions of Lives
Scientists have uncovered a way to make existing antifungal drugs work again against deadly, drug-resistant fungi. Fungal infections claim millions of lives worldwide each year, and current medical treatments are failing to keep pace. [...]
Scientists Trap Thyme’s Healing Power in Tiny Capsules
A new micro-encapsulation breakthrough could turn thyme’s powerful health benefits into safer, smarter nanodoses. Thyme extract is often praised for its wide range of health benefits, giving it a reputation as a natural medicinal [...]
Scientists Develop Spray-On Powder That Instantly Seals Life-Threatening Wounds
KAIST scientists have created a fast-acting, stable powder hemostat that stops bleeding in one second and could significantly improve survival in combat and emergency medicine. Severe blood loss remains the primary cause of death from [...]
Oceans Are Struggling To Absorb Carbon As Microplastics Flood Their Waters
New research points to an unexpected way plastic pollution may be influencing Earth’s climate system. A recent study suggests that microscopic plastic pollution is reducing the ocean’s capacity to take in carbon dioxide, a [...]
Molecular Manufacturing: The Future of Nanomedicine – New book from Frank Boehm
This book explores the revolutionary potential of atomically precise manufacturing technologies to transform global healthcare, as well as practically every other sector across society. This forward-thinking volume examines how envisaged Factory@Home systems might enable the cost-effective [...]
New Book! NanoMedical Brain/Cloud Interface – Explorations and Implications
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
Global Health Care Equivalency in the Age of Nanotechnology, Nanomedicine and Artificial Intelligence
A new book by Frank Boehm, NanoappsMedical Inc. Founder. This groundbreaking volume explores the vision of a Global Health Care Equivalency (GHCE) system powered by artificial intelligence and quantum computing technologies, operating on secure [...]
Miller School Researchers Pioneer Nanovanilloid-Based Brain Cooling for Traumatic Injury
A multidisciplinary team at the University of Miami Miller School of Medicine has developed a breakthrough nanodrug platform that may prove beneficial for rapid, targeted therapeutic hypothermia after traumatic brain injury (TBI). Their work, published in ACS [...]
COVID-19 still claims more than 100,000 US lives each year
Centers for Disease Control and Prevention researchers report national estimates of 43.6 million COVID-19-associated illnesses and 101,300 deaths in the US during October 2022 to September 2023, plus 33.0 million illnesses and 100,800 deaths [...]
Nanomedicine in 2026: Experts Predict the Year Ahead
Progress in nanomedicine is almost as fast as the science is small. Over the last year, we've seen an abundance of headlines covering medical R&D at the nanoscale: polymer-coated nanoparticles targeting ovarian cancer, Albumin recruiting nanoparticles for [...]