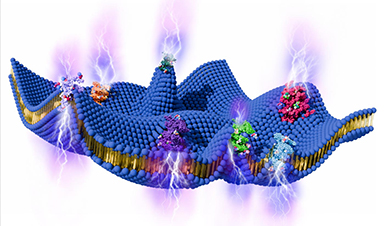
| Just because a model catalyst effectively drives a reaction in a well-controlled environment doesn’t mean it will work as well under more practical conditions. For years, scientists have strived to predict catalysts’ performance. In an ultrahigh vacuum model reactor, catalysts typically work at lower pressures and temperatures than in applied catalytic reactor studies. | |
| Now, a team devised a way to bridge the gap between these two extremes. Using their approach, they can predict catalyst performance across a wider range of temperatures and pressures (Nature Catalysis, “Crossing the great divide between single-crystal reactivity and actual catalyst selectivity with pressure transients”). | |
| The findings show that scientists can use fundamental surface science to predict behavior in applied catalytic reactor studies. The study is a stepping stone in designing efficient catalytic processes. Further, this work offers in-depth insight into a series of reactions that turn alcohols to higher-value chemicals. |
Image Credit: Ryan Chen, Lawrence Livermore National Laboratory
News This Week
COVID-19 still claims more than 100,000 US lives each year
Centers for Disease Control and Prevention researchers report national estimates of 43.6 million COVID-19-associated illnesses and 101,300 deaths in the US during October 2022 to September 2023, plus 33.0 million illnesses and 100,800 deaths [...]
Nanomedicine in 2026: Experts Predict the Year Ahead
Progress in nanomedicine is almost as fast as the science is small. Over the last year, we've seen an abundance of headlines covering medical R&D at the nanoscale: polymer-coated nanoparticles targeting ovarian cancer, Albumin recruiting nanoparticles for [...]
Lipid nanoparticles could unlock access for millions of autoimmune patients
Capstan Therapeutics scientists demonstrate that lipid nanoparticles can engineer CAR T cells within the body without laboratory cell manufacturing and ex vivo expansion. The method using targeted lipid nanoparticles (tLNPs) is designed to deliver [...]
The Brain’s Strange Way of Computing Could Explain Consciousness
Consciousness may emerge not from code, but from the way living brains physically compute. Discussions about consciousness often stall between two deeply rooted viewpoints. One is computational functionalism, which holds that cognition can be [...]
First breathing ‘lung-on-chip’ developed using genetically identical cells
Researchers at the Francis Crick Institute and AlveoliX have developed the first human lung-on-chip model using stem cells taken from only one person. These chips simulate breathing motions and lung disease in an individual, [...]
Cell Membranes May Act Like Tiny Power Generators
Living cells may generate electricity through the natural motion of their membranes. These fast electrical signals could play a role in how cells communicate and sense their surroundings. Scientists have proposed a new theoretical [...]
This Viral RNA Structure Could Lead to a Universal Antiviral Drug
Researchers identify a shared RNA-protein interaction that could lead to broad-spectrum antiviral treatments for enteroviruses. A new study from the University of Maryland, Baltimore County (UMBC), published in Nature Communications, explains how enteroviruses begin reproducing [...]
New study suggests a way to rejuvenate the immune system
Stimulating the liver to produce some of the signals of the thymus can reverse age-related declines in T-cell populations and enhance response to vaccination. As people age, their immune system function declines. T cell [...]
Nerve Damage Can Disrupt Immunity Across the Entire Body
A single nerve injury can quietly reshape the immune system across the entire body. Preclinical research from McGill University suggests that nerve injuries may lead to long-lasting changes in the immune system, and these [...]
Fake Science Is Growing Faster Than Legitimate Research, New Study Warns
New research reveals organized networks linking paper mills, intermediaries, and compromised academic journals Organized scientific fraud is becoming increasingly common, ranging from fabricated research to the buying and selling of authorship and citations, according [...]
Scientists Unlock a New Way to Hear the Brain’s Hidden Language
Scientists can finally hear the brain’s quietest messages—unlocking the hidden code behind how neurons think, decide, and remember. Scientists have created a new protein that can capture the incoming chemical signals received by brain [...]
Does being infected or vaccinated first influence COVID-19 immunity?
A new study analyzing the immune response to COVID-19 in a Catalan cohort of health workers sheds light on an important question: does it matter whether a person was first infected or first vaccinated? [...]
We May Never Know if AI Is Conscious, Says Cambridge Philosopher
As claims about conscious AI grow louder, a Cambridge philosopher argues that we lack the evidence to know whether machines can truly be conscious, let alone morally significant. A philosopher at the University of [...]
AI Helped Scientists Stop a Virus With One Tiny Change
Using AI, researchers identified one tiny molecular interaction that viruses need to infect cells. Disrupting it stopped the virus before infection could begin. Washington State University scientists have uncovered a method to interfere with a key [...]
Deadly Hospital Fungus May Finally Have a Weakness
A deadly, drug-resistant hospital fungus may finally have a weakness—and scientists think they’ve found it. Researchers have identified a genetic process that could open the door to new treatments for a dangerous fungal infection [...]
Fever-Proof Bird Flu Variant Could Fuel the Next Pandemic
Bird flu viruses present a significant risk to humans because they can continue replicating at temperatures higher than a typical fever. Fever is one of the body’s main tools for slowing or stopping viral [...]