Exciting and novel research has investigated the internalization of nanoparticles by cells to optimize drug delivery into target cells. This research, published within the journal, Pharmaceutics, aims to analyze the simultaneous uptake of two different types of nanoparticles into cells.
Enhancing Drug Delivery Using Nanoparticles
Nanoparticles, sized within the nanoscale, between 1 and 100 nm, hold high efficacy for drug delivery due to their ability to interact with biological systems.
The use of nanocarriers to carry drugs to their target cells is one of the advantages of incorporating nanotechnology within medicine, especially with their surface functionalization ability which enables them to target cells more effectively. This allows them to be more beneficial than the conventional method of drug delivery which has a systemic effect and can cause adverse effects on all cells rather than just targeting specific cells.
Understanding how many carriers enter cells is critical for deciphering the intercellular dose that the target cells are provided to determine the efficacy of utilizing nanoparticles in this way and advancing drug delivery systems.
While this area of research has already been spearheaded with previous research with internalization pathways for particles being investigated, the effect of cell uptake when two different particles are exposed to the cell simultaneously is limited.
These investigations include reports of an increase in cell uptake of gold and iron nanoparticles when both particles were exposed to murine macrophages. However, another researcher has described changes to cellular function with simultaneous exposure, with carbon black and iron oxide particles exposed to human epithelial cells, possibly resulting in protein and lipid oxidation.
Interestingly, this novel research can be useful when enhancing drug delivery in the body by using two different particles that can be used for various applications to increase the efficacy of drug treatment for diseases and disorders.
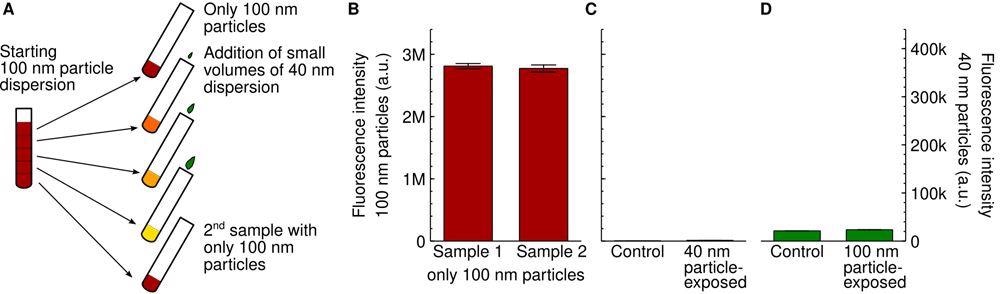
Experimental setup. (A) Schematic showing the dispersion procedure we used to ensure that the concentration of the 100 nm particles was the same for all samples. A first dispersion of the 100 nm particles was prepared and then divided up. To the resulting samples, different small volumes of a 40 nm particle dispersion were added. The first and last sample were left without 40 nm particles to serve as controls. Note that the colours are schematic only. (B) Nanoparticle fluorescence of cells exposed to the two different control dispersions of the 100 nm particles mentioned in panel A (first and last sample; 20 μg/mL or 0.060 nM). The similarity of the signal is consistent with the two control samples having the same nanoparticle concentration. (C,D) Lack of cross-talk between the two particle signals. (C) Cells were exposed to only the 40 nm particles (100 μg/mL; 4.7 nM) and the fluorescence intensity measured at the same wavelengths where researchers measured the 100 nm particles (left axis). There is a low background signal (right bar), but this is comparable to control cells not exposed to any particles at all (left bar). (D) Cells were exposed to only the 100 nm particles (80 μg/mL; 0.24 nM) and the fluorescence intensity measured at the same wavelengths where we measure the 40 nm particles (right axis). There is a low background signal (right bar), but this is comparable to control cells not exposed to any particles at all (left bar). © De Boer I, Richards CJ, Åberg C. (2022)
Innovative Research
The researchers of this study exposed HeLa (adenocarcinomic human cervical epithelial) cells to two different sized carboxylated polystyrene nanoparticles, including 40 nm and 100 nm diameter particles labeled with different fluorescent dyes to track the movement.
They observed that smaller particles had an increased cell uptake in the presence of larger-sized particles while larger particle uptake was impeded in the presence of smaller particles. This confirmed previous research on simultaneous cell uptake.
However, this increase was confirmed of cells within a medium with serum and the formation of a biomolecular corona on particles, which was not the same as previous research within a serum-free medium.
This research can enable the understanding of whether the competition between different concentrations of various sized particles is dependent on cell types; through the examination of 2D fluorescence distribution of the two measurable particles, the results have illustrated all cells take up both particles, but this may be within varying levels.
A limitation of this research is a lack of comprehension about the uptake mechanisms underlying the competitive nature of two various sized nanoparticles. With further research, the cellular mechanisms of this uptake can be clarified to understand these observations.
Significant Implications for Enhanced Drug Delivery
This research can be utilized to enhance drug delivery with the presence of a second nanoparticle, such as one of a larger size with the aim of increasing the cellular uptake of the first nanoparticle. The implications of this can be significant for increasing the intercellular drug dose found within cells and can be a start to advancing nanotechnology-based drug delivery systems.
With nanoparticles already being able to be functionalized through surface ligands, the use of these as nanocarriers for drug delivery as well as secondary particles can be an innovative approach to increasing the therapeutic selectivity of cells.
This can be utilized for diseases and even for cancer treatment to develop particles that work together for enhanced drug delivery into target cells, sustained delivery, and possibly the clearance of the drug from the body. Innovative drug delivery systems with more control through this approach may be a step towards personalized medicine that prioritizes patient care.
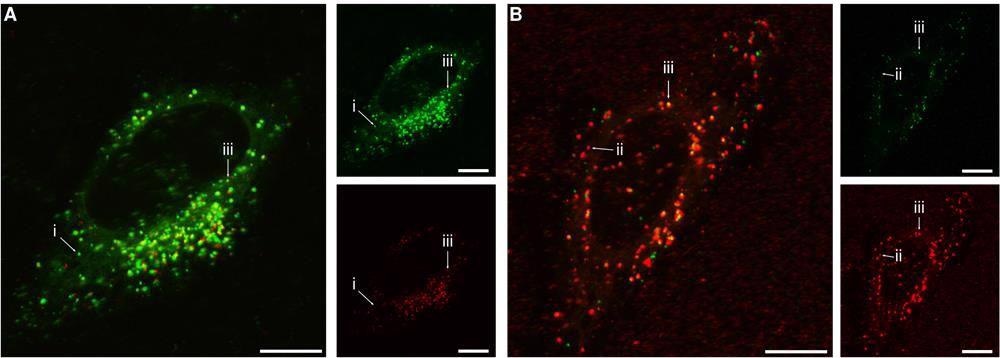
Subcellular distribution of the 40 nm and 100 nm particles. Cells were exposed for 24 h to both the 40 nm and 100 nm particles simultaneously and then observed using confocal fluorescence microscopy. (A) Concentration of 100 and 40 nm particles 20 and 100 μg/mL, respectively (0.060 nM and 4.7 nM; conditions correspond to the highest 40 nm particle concentration in Figures 2 and S3). (B) Concentration of 40 and 100 nm particles 6.25 and 80 μg/mL, respectively (0.30 and 0.24 nM; conditions correspond to the highest 100 nm particle concentration in Figures 4 and S4). The larger images show overlaps of both fluorescence colours, while the smaller images show the individual colours. (Green) 40 nm particles; (red) 100 nm particles. Arrows show examples of (i) 40 nm particle( s) (green) in the absence of 100 nm particles; (ii) 100 nm particle(s) (red) in the absence of 40 nm particles; (iii) 40 and 100 nm particles in the same location. The results show that the two particles often end up in the same location, but not always. All scale bars correspond to 10 μm. © De Boer I, Richards CJ, Åberg C. (2022)
News
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]