| The painful feeling of receiving an injection through a hypodermic needle or with the unpleasant sensation of swallowing a large pill is a globally familiar sensation. But what if a revolutionary and gentler way of administering drugs was in the works? | |
| For over two decades, researchers have been investigating various types of microneedles as a minimally invasive method for transdermal drug delivery. Arrays of microneedles can be designed to be loaded with a drug or chemical, which they then release over time onto the blood stream after piercing slightly beyond the skin layers. | |
| Microneedles offer several advantages compared to other types of drug delivery. First, they are painless and cause virtually no damage to the skin nor bleeding. Second, they can be self-administered. Third, unlike traditional needles, the disposal of microneedles is much easier as they don’t leave behind hazardous waste. | |
| Unfortunately, there are still a few challenges that need to be addressed before microneedles become the next big thing in healthcare. One is their fabrication cost, which generally involves expensive molds, materials, and machinery. | |
| Another issue is the aggregation and degradation of proteins when microneedles are pre-loaded with a protein-based medicine, as these molecules are quite sensitive to external conditions such as temperature, acidity and salt concentration. | |
| In a recent study published in Biomacromolecules (“Facile Photolithographic Fabrication of Zwitterionic Polymer Microneedle with Protein Aggregation Inhibition for Transdermal Drug Delivery”), two research teams from Japan and Thailand collaborated to address the main limitations of existing microneedles. | |
| On the other hand, Dr. Paisan Khanchaitit and his team at the National Science and Technology Development Agency (NANOTEC), Thailand, perfected a microneedle fabrication method suitable for the industrial scale based on photolithography. | |
| By combining these two efforts, the teams managed to produce microneedles patches with several attractive properties and potential scalability to clinical settings. | |
| The microneedles themselves are made of a non-degradable, biocompatible hydrogel that also contains zwitterionic poly-sulfobetaine (poly-SPB). As reported in previous studies by the same authors, this polymer suppresses protein aggregation. Thus, the researchers incorporated it during the fabrication process and showed that the proteins pre-loaded in the microneedles were stable even when subjected to various external stresses. | |
| Additionally, the scientists developed a straightforward and cost-effective way to fabricate microneedle arrays made from the abovementioned materials. They resorted to photolithography, a process in which a photomask is used to selectively block UV light from reaching a target surface to control chemical reactions locally, as Dr. Khanchaitit explains: “UV light passing through the photomask generates free radicals in the polymer resin during the fabrication process, resulting in photopolymerization and the subsequent formation of 3D microneedle structure patterns on a clear and flexible substrate.” | |
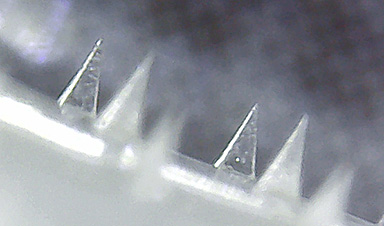
| This fabrication procedure requires inexpensive equipment only and takes about five minutes, yet produces four-point star-shaped microneedles with remarkable mechanical strength. | |
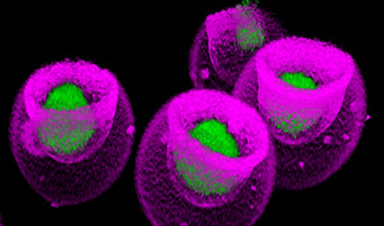
| To test the performance of these microneedle arrays for drug delivery, the researchers loaded them with 50 microliters of drug solutions containing rhodamine B as a dye alongside lysozyme and insulin as example proteins (see Figure above). | |
| Through various experiments on porcine skin, the teams verified that their microneedle patches offered both high drug-loading capacity and high drug-release rate. Moreover, they confirmed that the microneedles could both load and preserve various water-soluble drugs and proteins simultaneously, eliminating the need for refrigeration. | |
| Overall, the proposed microneedle arrays seem to be a remarkably promising platform for administering therapeutic drugs and vaccines, as Prof. Matsumura concludes: “The superior characteristics of our microneedles may change the way drugs are administered and could enable the development and delivery of advanced protein-based drugs for the treatment of various disorders.” Who knows what other revolutionary innovations in medicine wait for us in the future? |
News
Completely New Use Discovered – This Traditional Herb Has Remarkable Nerve Regenerative Properties
Blessed thistle (Cnicus benedictus), a member of the Asteraceae family, thrives in our climate. This plant has been utilized for centuries as a medicinal herb, often consumed as an extract or tea to support [...]
Scientists study lipids cell by cell, making new cancer research possible
Imagine being able to look inside a single cancer cell and see how it communicates with its neighbors. Scientists are celebrating a new technique that lets them study the fatty contents of cancer cells, [...]
Antibiotic Breakthrough: Revolutionary Chinese Study Paves Way for Superbug Defeating Drugs
New research reveals that fluorous lipopetides act as highly effective antibiotics. Bacterial infections resistant to multiple drugs, which no existing antibiotics can treat, represent a significant worldwide challenge. A research group from China has [...]
Signs of Multiple Sclerosis Show Up in Blood Years Before Symptoms Appear
UCSF scientists clear a potential path toward earlier treatment for a disease that affects nearly 1,000,000 people in the United States. By Levi Gadye In a discovery that could hasten treatment for patients with multiple [...]
Advanced RNA Sequencing Reveals the Drivers of New COVID Variants
A study reveals that a new sequencing technique, tARC-seq, can accurately track mutations in SARS-CoV-2, providing insights into the rapid evolution and variant development of the virus. The SARS-CoV-2 virus that causes COVID has the unsettling [...]
No More Endless Boosters? Scientists Develop One-for-All Virus Vaccine
End of the line for endless boosters? Researchers at UC Riverside have developed a new vaccine approach using RNA that is effective against any strain of a virus and can be used safely even by babies or the immunocompromised. Every [...]
How Are Hydrogels Shaping the Future of Biomedicine?
Hydrogels have gained widespread recognition and utilization in biomedical engineering, with their applications dating back to the 1960s when they were first used in contact lens production. Hydrogels are distinguished from other biomaterials in [...]
Nanovials method for immune cell screening uncovers receptors that target prostate cancer
A recent UCLA study demonstrates a new process for screening T cells, part of the body's natural defenses, for characteristics vital to the success of cell-based treatments. The method filters T cells based on [...]
New Research Reveals That Your Sense of Smell May Be Smarter Than You Think
A new study published in the Journal of Neuroscience indicates that the sense of smell is significantly influenced by cues from other senses, whereas the senses of sight and hearing are much less affected. A popular [...]
Deadly bacteria show thirst for human blood: the phenomenon of bacterial vampirism
Some of the world's deadliest bacteria seek out and feed on human blood, a newly-discovered phenomenon researchers are calling "bacterial vampirism." A team led by Washington State University researchers has found the bacteria are [...]
Organ Architects: The Remarkable Cells Shaping Our Development
Finding your way through the winding streets of certain cities can be a real challenge without a map. To orient ourselves, we rely on a variety of information, including digital maps on our phones, [...]
Novel hydrogel removes microplastics from water
Microplastics pose a great threat to human health. These tiny plastic debris can enter our bodies through the water we drink and increase the risk of illnesses. They are also an environmental hazard; found [...]
Researchers Discover New Origin of Deep Brain Waves
Understanding hippocampal activity could improve sleep and cognition therapies. Researchers from the University of California, Irvine’s biomedical engineering department have discovered a new origin for two essential brain waves—slow waves and sleep spindles—that are critical for [...]
The Lifelong Cost of Surviving COVID: Scientists Uncover Long-Term Effects
Many of the individuals released to long-term acute care facilities suffered from conditions that lasted for over a year. Researchers at UC San Francisco studied COVID-19 patients in the United States who survived some of the longest and [...]
Previously Unknown Rogue Immune Key to Chronic Viral Infections Discovered
Scientists discovered a previously unidentified rogue immune cell linked to poor antibody responses in chronic viral infections. Australian researchers have discovered a previously unknown rogue immune cell that can cause poor antibody responses in [...]
Nature’s Betrayal: Unmasking Lead Lurking in Herbal Medicine
A case of lead poisoning due to Ayurvedic medicine use demonstrates the importance of patient history in diagnosis and the need for public health collaboration to prevent similar risks. An article in CMAJ (Canadian Medical Association [...]