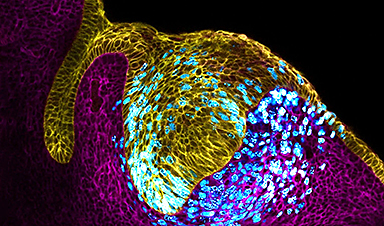
Finding your way through the winding streets of certain cities can be a real challenge without a map. To orient ourselves, we rely on a variety of information, including digital maps on our phones, as well as recognizable shops and landmarks. Cells in our bodies face a similar problem when building our organs during embryogenesis. They need instructions on where to go and how to behave. Luckily, like cell phone towers in a city, embryos feature special cells in specific locations, known as organizers, that send signals to other cells and help them organize to build our complex organs.
Some of these signals are molecules sent from the organizer, a privileged signaling center. Cells around it receive stronger or weaker signals depending on their location, and they take decisions accordingly. Errors in the location of these messaging centers in the tissue lead to embryonic malformations that can be fatal. Scientists have known the relevance of these signaling centers for a long time, but how these appear at specific locations remained elusive.
Discovery Through International Collaboration
It took an international collaboration of physicists and biologists to pinpoint the answer. Several years ago, the laboratories of Prof. Ophir Klein at Cedars-Sinai Guerin Children’s and the University of California, San Francisco (UCSF), and Prof. Otger Campàs at the Physics of Life Excellence Cluster of TU Dresden and the University of California, Santa Barbara (UCSB), had a hint of how it may work and joined forces. Together, they figured out that it is the mechanical pressure inside the growing tissue that dictates where the signaling center will emerge.
“Our work shows that both mechanical pressure and molecular signaling play a role in organ development,” said Ophir Klein, MD, PhD, Executive Director of Cedars-Sinai Guerin Children’s, where he is also the David and Meredith Kaplan Distinguished Chair in Children’s Health, and co-corresponding author of the study.
Mechanical Pressure in Organizing Cells
The study, published in Nature Cell Biology, shows that as cells grow in the embryonic incisor tooth, they feel the growing pressure and use this information to organize themselves. “It’s like those toys that absorb water and grow in size,” said Neha Pincha Shroff, PhD, a postdoctoral scholar in the School of Dentistry at UCSF, and co-first author of the study. “Just imagine that happening in a confined space. What happens in the incisor knot is that the cells multiply in number in a fixed space and this causes a pressure to build up at the center, which then becomes a cluster of specialized cells.” Like people in a crowded bar, cells in the tissue start feeling the squeeze from their peers. The researchers found that the cells feeling the stronger pressure stop growing and start sending signals to organize the other surrounding cells in the tooth. They were literally pressed into becoming the tooth organizer.
“We were able to use microdroplet techniques that our lab previously developed to figure out how the buildup of mechanical pressure affects organ formation,” said co-corresponding author of the study Otger Campàs, Ph.D., who is currently Managing Director, Professor and Chair of Tissue Dynamics at the Physics of Life Excellence Cluster of TU Dresden, and former Associate Professor of Mechanical Engineering at UCSB. “It is really exciting that tissue pressure has a role in establishing signaling centers. It will be interesting to see if or how mechanical pressure affects other important developmental processes.”
Embryos use several of these signaling centers to guide cells as they form tissues and organs. Like building skyscrapers or bridges, sculpting our organs involves tight planning, a lot of coordination, and the right structural mechanics. Failure in any of these processes can be catastrophic when it comes to building a bridge, and it can also be damaging for us when growing in the womb.
“By understanding how an embryo forms organs, we can start to ask questions about what goes wrong in children born with congenital malformations,” said Ophir Klein. “This work may lead to additional research into how birth defects are formed and can be prevented.”
Reference: “Proliferation-driven mechanical compression induces signalling centre formation during mammalian organ development” by Neha Pincha Shroff, Pengfei Xu, Sangwoo Kim, Elijah R. Shelton, Ben J. Gross, Yucen Liu, Carlos O. Gomez, Qianlin Ye, Tingsheng Yu Drennon, Jimmy K. Hu, Jeremy B. A. Green, Otger Campàs and Ophir D. Klein, 3 April 2024, Nature Cell Biology.
DOI: 10.1038/s41556-024-01380-4
The study was funded by the National Institute of Dental and Craniofacial Research (OK and OC) in the USA, the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy, and the Cluster of Excellence Physics of Life of TU Dresden (OC).
News
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]
Controlling This One Molecule Could Halt Alzheimer’s in Its Tracks
New research identifies the immune molecule STING as a driver of brain damage in Alzheimer’s. A new approach to Alzheimer’s disease has led to an exciting discovery that could help stop the devastating cognitive decline [...]