Research from Weill Cornell Medicine reveals that astrocyte receptors impact cognitive functions differently in males and females, suggesting a need for sex-specific approaches in developing treatments targeting these brain cells.
Scientists at Weill Cornell Medicine have discovered the first evidence that receptors in astrocytes, brain cells that support and regulate neurons, can have contrasting effects on cognitive function in male and female preclinical models. This research highlights the role of astrocytes in contributing to gender-specific brain mechanisms.
While many studies have tested the behavioral effects of astrocytic receptors, none of them have addressed whether biological sex plays a role and most have tested only males. This study, published on May 24 in Cell Reports, challenges the long-standing assumption that astrocytic signaling has similar cognitive effects in both sexes.
“Our study reveals that previously reported cognitive effects in males can’t be extrapolated to females,” said Dr. Anna G. Orr, the Nan and Stephen Swid Assistant Professor of Frontotemporal Dementia Research and an assistant professor of neuroscience in the Feil Family Brain and Mind Research Institute and the Helen and Robert Appel Alzheimer’s Research Institute at Weill Cornell Medicine.
Changes in astrocytic receptors are seen in various neurological conditions with known sex differences, including neurodegenerative disorders, schizophrenia, stroke, and epilepsy. However, the mechanisms promoting sex differences remain poorly understood.
How do Male and Female Brains Differ?
In the study, Dr. Samantha M. Meadows, first author and former graduate student in the Orr lab, focused on mGluR3, a predominant glutamate receptor in astrocytes and a top-altered gene in dementia. The team used gene editing and stimulation of engineered receptors in animal models to selectively manipulate astrocytes and examine the effects of mGluR3 and related receptors on learning, memory, and other cognitive and behavioral outcomes.
The researchers found that increasing astrocytic mGluR3 levels enhanced memory in older females and reducing these levels was sufficient to impair memory in young females, demonstrating that mGluR3 promotes memory recall in females. However, in males, reducing mGluR3 enhanced memory, and increasing the receptor had no effects. “Interestingly, the cognitive impact of these receptors is not conserved among sexes,” Dr. Meadows said.
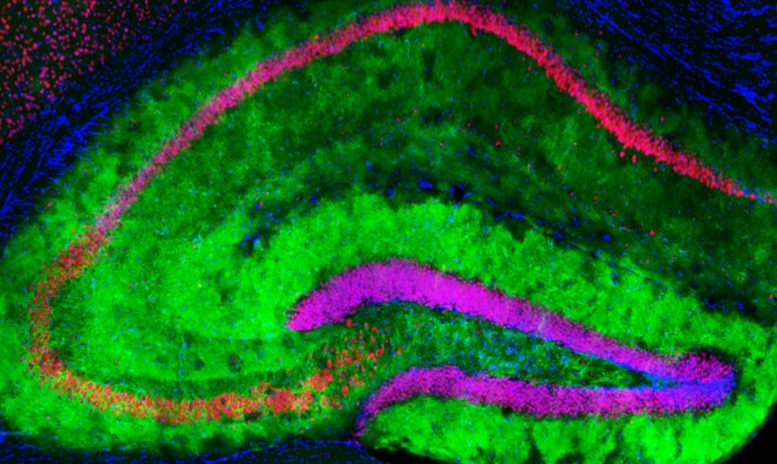
This image of the mouse hippocampus, a part of the brain involved in learning and memory, shows mGluR3 receptors on astrocytes (green), neurons (red), and cell nuclei (blue). Credit: Orr Lab
To understand if these divergent effects were unique to mGluR3 or reflected a broader feature of astrocytic receptor signaling, Dr. Meadows worked with co-author Dr. Adam L. Orr, an assistant professor of research in neuroscience in the Brain and Mind Research Institute and the Appel Alzheimer’s Disease Research Institute, to selectively stimulate different astrocytic receptors while mice performed tasks involving learning and memory.
To their surprise, the team found further evidence that receptor activation caused either memory enhancement or impairment, depending on biological sex. “Normal brain function seems to require a sex-specific balance in astrocytic signaling,” Dr. Adam Orr said.
This study suggests that mGluR3 modulators being developed for treating disorders such as schizophrenia and anxiety may need further study to assess their impact on different sexes. “Therapeutics influencing astrocytic receptors may cause sex-specific cognitive effects in part due to the divergent roles of astrocytes in males and females,” said Dr. Anna Orr.
The lab is investigating what may cause the differential effects and if other brain functions are also changed in a sex-specific way.
Reference: “Hippocampal astrocytes induce sex-dimorphic effects on memory” by Samantha M. Meadows, Fernando Palaguachi, Minwoo Wendy Jang, Avital Licht-Murava, Daniel Barnett, Till S. Zimmer, Constance Zhou, Samantha R. McDonough, Adam L. Orr and Anna G. Orr, 24 May 2024, Cell Reports.
DOI: 10.1016/j.celrep.2024.114278
News
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]