Should we be prepared to change the population composition of a species in order to wipe out a disease that is a terrible burden to mankind? During a well-attended working breakfast organised by the European Parliament’s Panel for the Future of Science and Technology (STOA) on 19 March 2019, experts and citizens delved into the case study of eradicating malaria by applying gene-drive technology. This genetic tool could enable us to suppress mosquito populations that transmit malaria by reducing the number of females. This would be done by introducing in some mosquitos a genetic mechanism that easily spreads – ‘drives’ – through the whole population over generations.
The purpose of the event was to gain insight into the science and ethics of gene-drive technology, and of genome-editing technologies in general. The meeting was chaired by Kay Swinburne, (ECR, UK) a STOA Panel member, who underlined the importance of invigorating public debate on such technologies in her opening statement. With more than 200 million cases of malaria each year worldwide, of which over 400 000 are fatal, no one doubts the importance of fighting this disease. This, as Kay Swinburne explained, makes the case less controversial than, for instance, human genome editing, and therefore it provides a good opportunity to focus on understanding the benefits of genetic technology. Such an understanding, combined with knowledge of the risks and concerns, and with awareness of different stakeholder perspectives, should help policy-makers anticipate the application of genome-editing technologies. With three expert presentations and a debate, this event provided input on all these fronts.
First, Jens Van Steerteghem, of KU Leuven, and a former STOA trainee, gave the audience a technical overview of gene-drive technology in the context of eradicating malaria. His presentation was based on a scientific briefing that was used in a preliminary foresight analysis project on gene drive and malaria. This project aimed to map the potential societal impact of gene-drive technology and exposed the need for a general risk assessment framework for biotechnological applications. Jens Steerteghem explained how the number of female mosquitos would be suppressed if the gene-drive method were applied: by introducing in males a gene on the Y chromosome that cuts their X chromosome, so that they only pass Y chromosomes to their offspring. This offspring are consequently exclusively male, and additionally carry the new gene on their Y chromosome.
Delphine Thizy, Stakeholder Engagement Manager with the non-profit research organisation Target Malaria delivered the second presentation. This organisation is developing the gene-drive technology method to reduce malaria. Delphine Thizy described the current state of the research at her organisation, commenting that only in September 2018, their team published a paper on a successful eradication experiment on a mosquito population in a containment cage. She also addressed some of the misconceptions and concerns she hears when explaining Target Malaria’s plans. These worries are expressed both by citizens in Western countries but also, and more importantly, from stakeholders in countries in sub-Saharan Africa that suffer from malaria. People question, for example, if the targeted mosquito species are crucial pollinators, or if the reduction of mosquitos would disturb food chains. For both cases, she indicated that there is no need to worry: the targeted mosquitoes are not known to be pollinators and just three mosquito species would be targeted out of 830 species in Africa alone.

NanoApps Medical, Inc. Aims To Develop Nanobiosensor for Malaria, Ebola, and Zika
 NanoApps Medical, Inc. (Vancouver, Canada) is working to develop a point of care diagnostic nanobiosensor platform for the detection of Malaria via saliva samples, which may be reconfigured to detect Ebola, and Zika. The use of this nanobiosensor will be far less invasive and safer than through the extraction of blood samples, while conveying more rapid results. The current gold standard for the detection of Malaria is blood smear microscopy, the results of which may take from many hours to several days to determine. This test also has the requirements of technical expertise in blood sample preparation, and a trained microscopist. Hence, the proposed nanobiosensor would be a significant improvement in terms of expediting and simplifying the diagnosis of Malaria, Ebola, and Zika in that it would be easy to administer, and provide rapid and clearly understandable results.
NanoApps Medical, Inc. (Vancouver, Canada) is working to develop a point of care diagnostic nanobiosensor platform for the detection of Malaria via saliva samples, which may be reconfigured to detect Ebola, and Zika. The use of this nanobiosensor will be far less invasive and safer than through the extraction of blood samples, while conveying more rapid results. The current gold standard for the detection of Malaria is blood smear microscopy, the results of which may take from many hours to several days to determine. This test also has the requirements of technical expertise in blood sample preparation, and a trained microscopist. Hence, the proposed nanobiosensor would be a significant improvement in terms of expediting and simplifying the diagnosis of Malaria, Ebola, and Zika in that it would be easy to administer, and provide rapid and clearly understandable results.![]()
News This Week
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
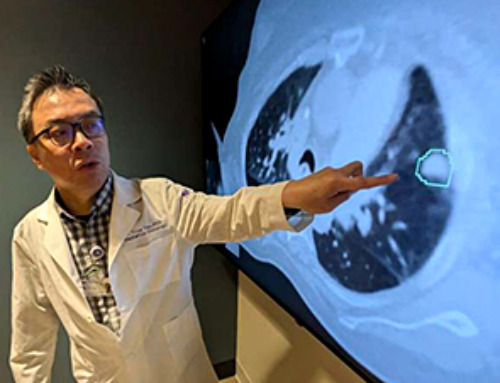
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]
















Leave A Comment