esearchers have detailed a mechanism in the distinctive corona of Covid-19 that could help scientists to rapidly find new treatments for the virus, and quickly test whether existing treatments are likely to work with mutated versions as they develop.
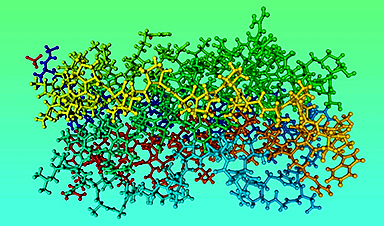
The team, led by the University of Warwick as part of the EUTOPIA community of European universities, have simulated movements in nearly 300 protein structures of the Covid-19 virus spike protein by using computational modelling techniques, in an effort to help identify promising drug targets for the virus.
In a new paper published today (19 February) in the journal Scientific Reports, the team of physicists and life scientists detail the methods they used to model the flexibility and dynamics of all 287 protein structures for the Covid-19 virus, also known as SARS-CoV-2, identified so far. Just like organisms, viruses are composed of proteins, large biomolecules that perform a variety of functions. The scientists believe that one method for treating the virus could be interfering with the mobility of those proteins.
They have made their data, movies and structural information, detailing how the proteins move and how they deform, for all 287 protein structures for Covid-19 that were available at the time of the study, publicly accessible to allow others to investigate potential avenues for treatments.
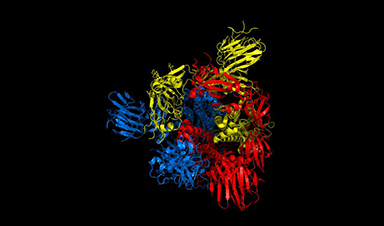
The researchers focused particular efforts on a part of the virus known as the spike protein, also called the Covid-19 echo domain structure, which forms the extended corona that gives coronaviruses their name. This spike is what allows the virus to attach itself to the ACE2 enzyme in human cell membranes, through which it causes the symptoms of Covid-19.
The spike protein is in fact a homotrimer, or three of the same type of protein combined. By modelling the movements of the proteins in the spike, the researchers identified a ‘hinge’ mechanism that allows the spike to hook onto a cell, and also opens up a tunnel in the virus that is a likely means of delivering the infection to the hooked cell. The scientists suggest that by finding a suitable molecule to block the mechanism – literally, by inserting a suitably sized and shaped molecule – pharmaceutical scientists will be able to quickly identify existing drugs that could be effective against the virus.
Lead author Professor Rudolf Roemer from the Department of Physics at the University of Warwick, who conducted the work while on a sabbatical at CY Cergy-Paris Université, said: “Knowing how this mechanism works is one way in which you can stop the virus, and in our study we are the first to see the detailed movement of opening. Now that you know what the range of this movement is, you can figure out what can block it.
“All those people who are interested in checking whether the protein structures in the virus could be drug targets should be able to examine this and see if the dynamics that we compute are useful to them.
“We couldn’t look closely at all the 287 proteins though in the time available. People should use the motion that we observe as a starting point for their own development of drug targets. If you find an interesting motion for a particular protein structure in our data, you can use that as the basis for further modelling or experimental studies.”
To investigate the proteins’ movements, the scientists used a protein flexibility modelling approach…
Image Credit: University of Warwick
Post by Amanda Scott, NA CEO. Follow her on twitter @tantriclens
Thanks to Heinz V. Hoenen. Follow him on twitter: @HeinzVHoenen
News
Breakthrough in Antimicrobial Technology with Cinnamon-Based Nanokiller
The need for innovative antimicrobial agents has become increasingly urgent due to the rise of antibiotic-resistant pathogens and the persistent threat of infections acquired during hospital stays. Traditional antibiotics and antiseptics are often ineffective [...]
The Silent Battle Within: How Your Organs Choose Between Mom and Dad’s Genes
Research reveals that selective expression of maternal or paternal X chromosomes varies by organ, driven by cellular competition. A new study published today (July 26) in Nature Genetics by the Lymphoid Development Group at the MRC [...]
Study identifies genes increasing risk of severe COVID-19
Whether or not a person becomes seriously ill with COVID-19 depends, among other things, on genetic factors. With this in mind, researchers from the University Hospital Bonn (UKB) and the University of Bonn, in [...]
Small regions of the brain can take micro-naps while the rest of the brain is awake and vice versa
Sleep and wake: They're totally distinct states of being that define the boundaries of our daily lives. For years, scientists have measured the difference between these instinctual brain processes by observing brain waves, with [...]
Redefining Consciousness: Small Regions of the Brain Can Take Micro-Naps While the Rest of the Brain Is Awake
The study broadly reveals how fast brain waves, previously overlooked, establish fundamental patterns of sleep and wakefulness. Scientists have developed a new method to analyze sleep and wake states by detecting ultra-fast neuronal activity [...]
AI Reveals Health Secrets Through Facial Temperature Mapping
Researchers have found that different facial temperatures correlate with chronic illnesses like diabetes and high blood pressure, and these can be detected using AI with thermal cameras. They highlight the potential of this technology [...]
Breakthrough in aging research: Blocking IL-11 extends lifespan and improves health in mice
In a recent study published in the journal Nature, a team of researchers used murine models and various pharmacological and genetic approaches to examine whether pro-inflammatory signaling involving interleukin (IL)-11, which activates signaling molecules such [...]
Promise for a universal influenza vaccine: Scientists validate theory using 1918 flu virus
New research led by Oregon Health & Science University reveals a promising approach to developing a universal influenza vaccine—a so-called "one and done" vaccine that confers lifetime immunity against an evolving virus. The study, [...]
New Projects Aim To Pioneer the Future of Neuroscience
One study will investigate the alterations in brain activity at the cellular level caused by psilocybin, the psychoactive substance found in “magic mushrooms.” How do neurons respond to the effects of magic mushrooms? What [...]
Decoding the Decline: Scientific Insights Into Long COVID’s Retreat
Research indicates a significant reduction in long COVID risk, largely due to vaccination and the virus’s evolution. The study analyzes data from over 441,000 veterans, showing lower rates of long COVID among vaccinated individuals compared [...]
Silicon Transformed: A Breakthrough in Laser Nanofabrication
A new method enables precise nanofabrication inside silicon using spatial light modulation and laser pulses, creating advanced nanostructures for potential use in electronics and photonics. Silicon, the cornerstone of modern electronics, photovoltaics, and photonics, [...]
Caught in the actinium: New research could help design better cancer treatments
The element actinium was first discovered at the turn of the 20th century, but even now, nearly 125 years later, researchers still don't have a good grasp on the metal's chemistry. That's because actinium [...]
Innovative Light-Controlled Drugs Could Revolutionize Neuropathic Pain Treatment
A team of researchers from the Institute for Bioengineering of Catalonia (IBEC) has developed light-activated derivatives of the anti-epileptic drug carbamazepine to treat neuropathic pain. Light can be harnessed to target drugs to specific [...]
Green Gold: Turning E-Waste Into a Treasure Trove of Rare Earth Metals
Scientists are developing a process inspired by nature that efficiently recovers europium from old fluorescent lamps. The approach could lead to the long-awaited recycling of rare earth metals. A small molecule that naturally serves [...]
Cambridge Study: AI Chatbots Have an “Empathy Gap,” and It Could Be Dangerous
A new study suggests a framework for “Child Safe AI” in response to recent incidents showing that many children perceive chatbots as quasi-human and reliable. A study has indicated that AI chatbots often exhibit [...]
Nanoparticle-based delivery system could offer treatment for diabetics with rare insulin allergy
Up to 3% of people with diabetes have an allergic reaction to insulin. A team at Forschungszentrum Jülich has now studied a method that could be used to deliver the active substance into the [...]