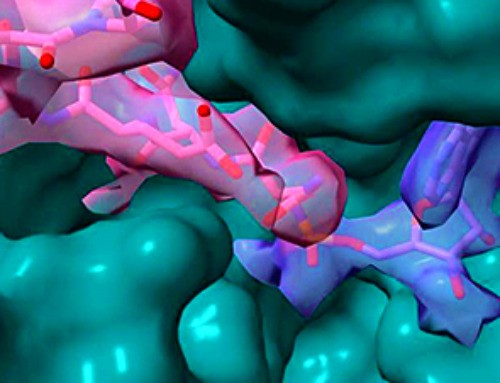
Lithium-ion batteries are the ultimate benchmark when it comes to mobile phones, tablet devices, and electric cars. Their storage capacity and power density are far superior to other rechargeable battery systems. Despite all the progress that has been made, however, smartphone batteries only last a day and electric cars need hours to be recharged. Scientists are therefore working on ways to improve the power densities and charging rates of all-round batteries.
“An important factor is the anode material,” explains Dina Fattakhova-Rohlfing from the Institute of Energy and Climate Research (IEK-1).
“In principle, anodes based on tin dioxide can achieve much higher specific capacities, and therefore store more energy, than the carbon anodes currently being used. They have the ability to absorb more lithium ions,” says Fattakhova-Rohlfing. “Pure tin oxide, however, exhibits very weak cycle stability – the storage capability of the batteries steadily decreases and they can only be recharged a few times. The volume of the anode changes with each charging and discharging cycle, which leads to it crumbling.”
One way of addressing this problem is hybrid materials or nanocomposites – composite materials that contain nanoparticles. The scientists developed a material comprising tin oxide nanoparticles enriched with antimony, on a base layer of graphene (“Making Ultrafast High-Capacity Anodes for Lithium-Ion Batteries via Antimony Doping of Nanosized Tin Oxide/Graphene Composites“).
The graphene basis aids the structural stability and conductivity of the material. The tin oxide particles are less than three nanometres in size and are directly “grown” on the graphene. The small size of the particle and its good contact with the graphene layer also improves its tolerance to volume changes – the lithium cell becomes more stable and lasts longer.
Image Credit: Prof. Dina Fattakhova-Rohlfing
News This Week
Team finds flawed data in recent study relevant to coronavirus antiviral development
The COVID pandemic illustrated how urgently we need antiviral medications capable of treating coronavirus infections. To aid this effort, researchers quickly homed in on part of SARS-CoV-2's molecular structure known as the NiRAN domain—an [...]
Drug-Coated Neural Implants Reduce Immune Rejection
Summary: A new study shows that coating neural prosthetic implants with the anti-inflammatory drug dexamethasone helps reduce the body’s immune response and scar tissue formation. This strategy enhances the long-term performance and stability of electrodes [...]
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]











Leave A Comment