Several difficulties have been associated with developing robust nanoscale coating on the surface of electrospun nanofibers. In a recent study published in Nature Communications, scientists have successfully produced a facile, controllable, and versatile method to develop superlubricated nano-skin (SLNS) on the single electrospun nanofiber in situ.
Electrospun Nanofibers
Nanofibers synthesized through electrospinning are commonly applied in several areas, including biomedical engineering, energy, and the environment. This is because electrospinning is responsible for producing highly controllable structures with specific functions.
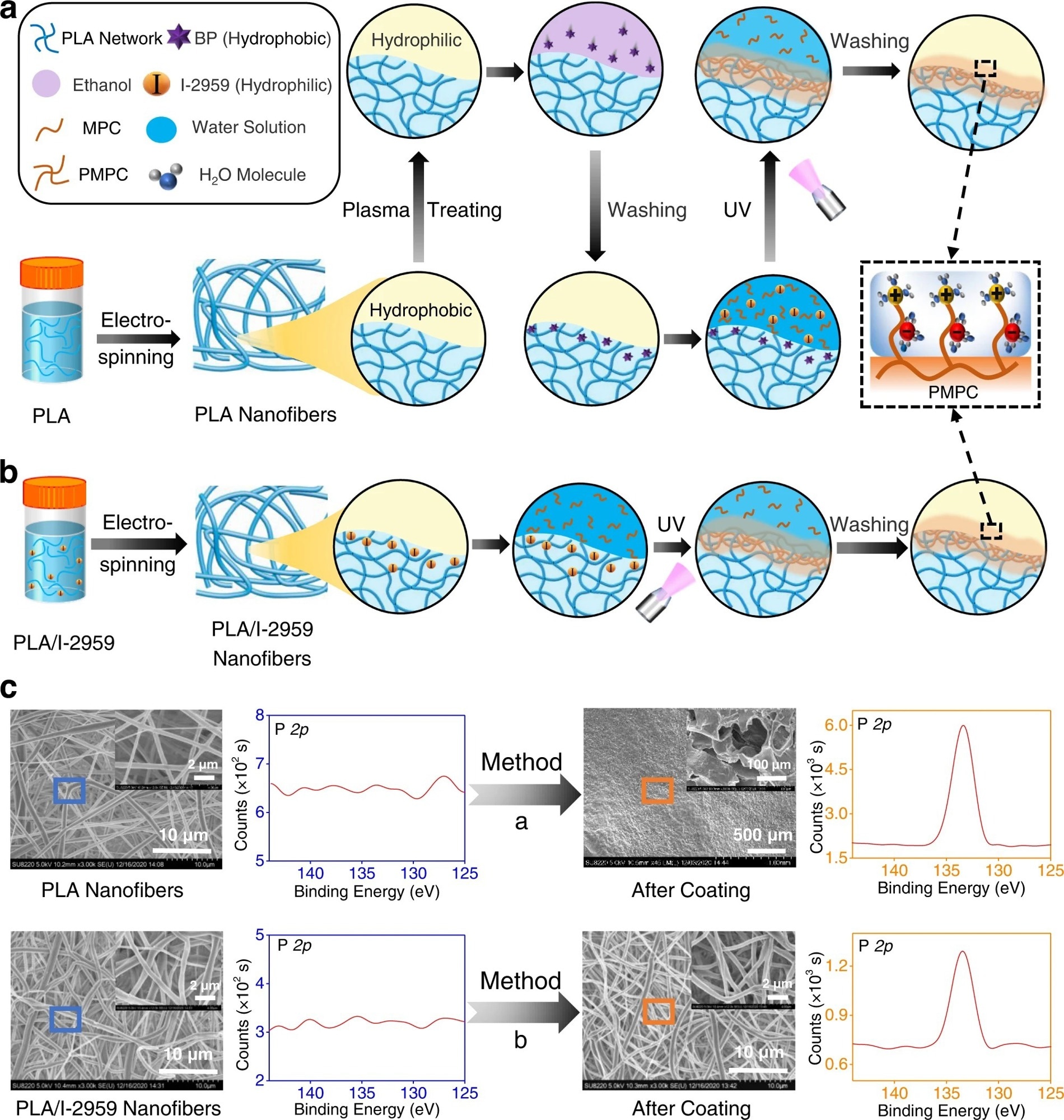
Figure 1. The comparison of our developed surface coating strategy to construct superlubricated nano-skin on electrospun nanofibers with previously reported method. a Schematic diagram of a slightly changed procedure from previous method17,18. PLA polylactic acid, BP benzophenone, MPC 2-methacryloyloxyethyl phosphorylcholine, PMPC poly MPC, UV ultraviolet. b Schematic diagram of our optimized method of subsurface-initiated polymerization. c Representative SEM and XPS results of electrospun nanofibers for the comparison of surface morphology and elemental composition between the method in a and in b. The experiments are replicated three times independently with similar results. © Wang, Y., Xu, Y., Zhai, W. et al. (2022)
These nanofibrous membranes are also used for in vivo treatments that require direct human tissue contact. The effectiveness of the application of electrospun nanofibers depends on their surface performance. The electrospun nanofiber’s surface properties, such as patterned structure and fiber orientation, are effectively adjusted via various methods.
The incorporation of these properties improves the cell growth capacity on the fiber surface along with cellular adhesion, which plays an important role in the acceleration of the tissue regeneration process. Nevertheless, uncontrolled cell growth and adhesion to adjacent tissues lead to irretrievable consequences.
Non-Specific Cell Adhesion Property of Electrospun Membranes
Electrospun polylactic acid (PLA)-based membranes (e.g., DK-film) are clinically used for anti-adhesion purposes. This membrane forms a barrier between the injured tissues. However, one of the disadvantages of this membrane is that it adheres to the tissue surface. Therefore, it is extremely crucial to create an electrospun membrane with superior non-adhesive surface properties to prevent post-operative adhesion.
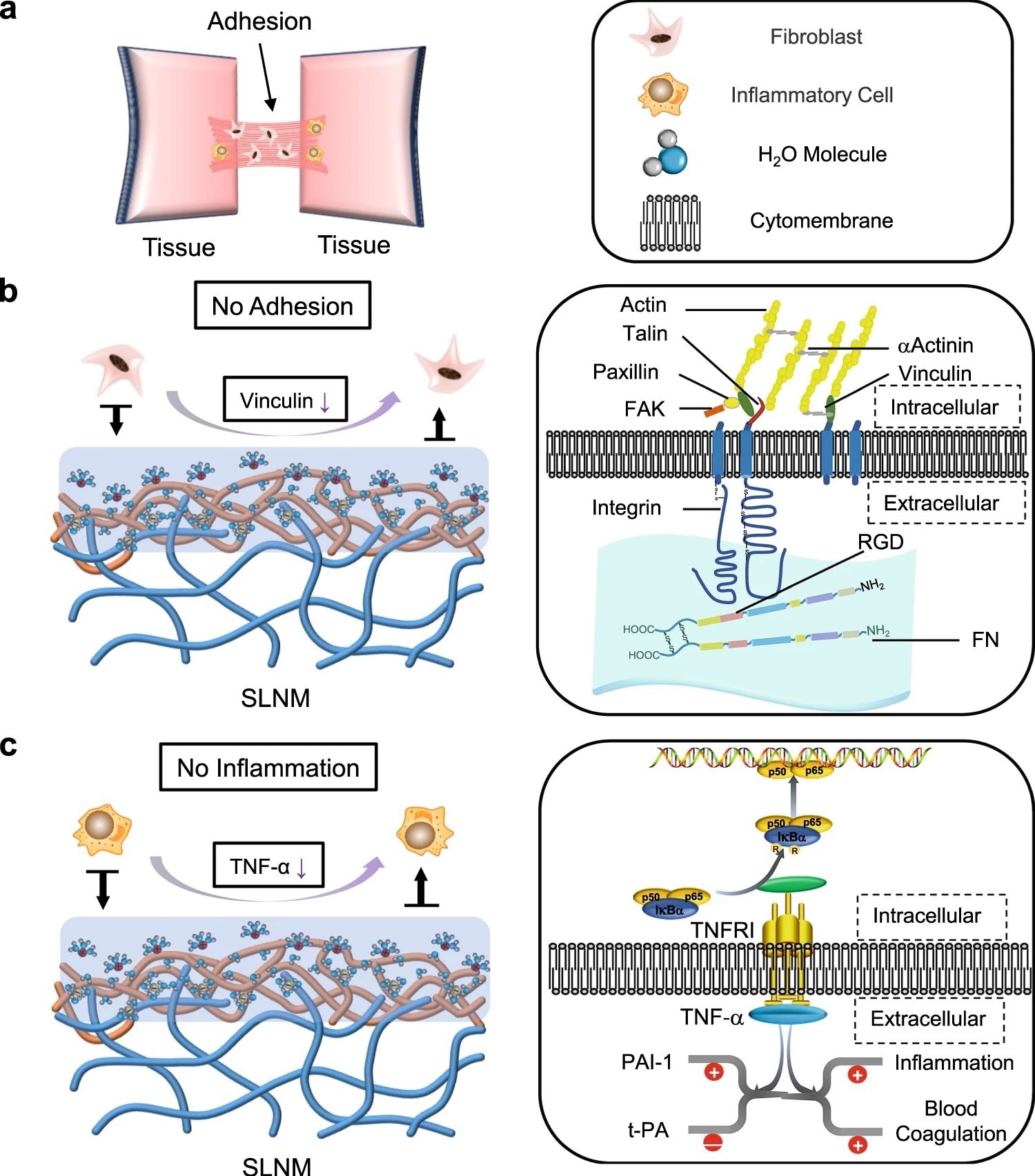
Hydration lubrication could be effectively used to develop nanofibrous membranes with non-adhesive surfaces. Mechanistically, polyelectrolyte polymers (e.g., poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC)) exhibit strong adsorption to the hydration layer due to the zwitterionic charges and, subsequently, promote an extremely low coefficient of friction (COF) between the sliding surfaces. This condition prevents non-specific cell adhesion.
Optimization of the interfacial bonding between the substrate polymer chains of nanofibers and the zwitterionic polymer chains in the coating is a challenging task. Although surface modification methods (e.g., grafting polymer chains) are used for this purpose, they cannot develop strong zwitterionic coatings. This is because PLA is sensitive to organic solvents and gets dissolved during surface modification.
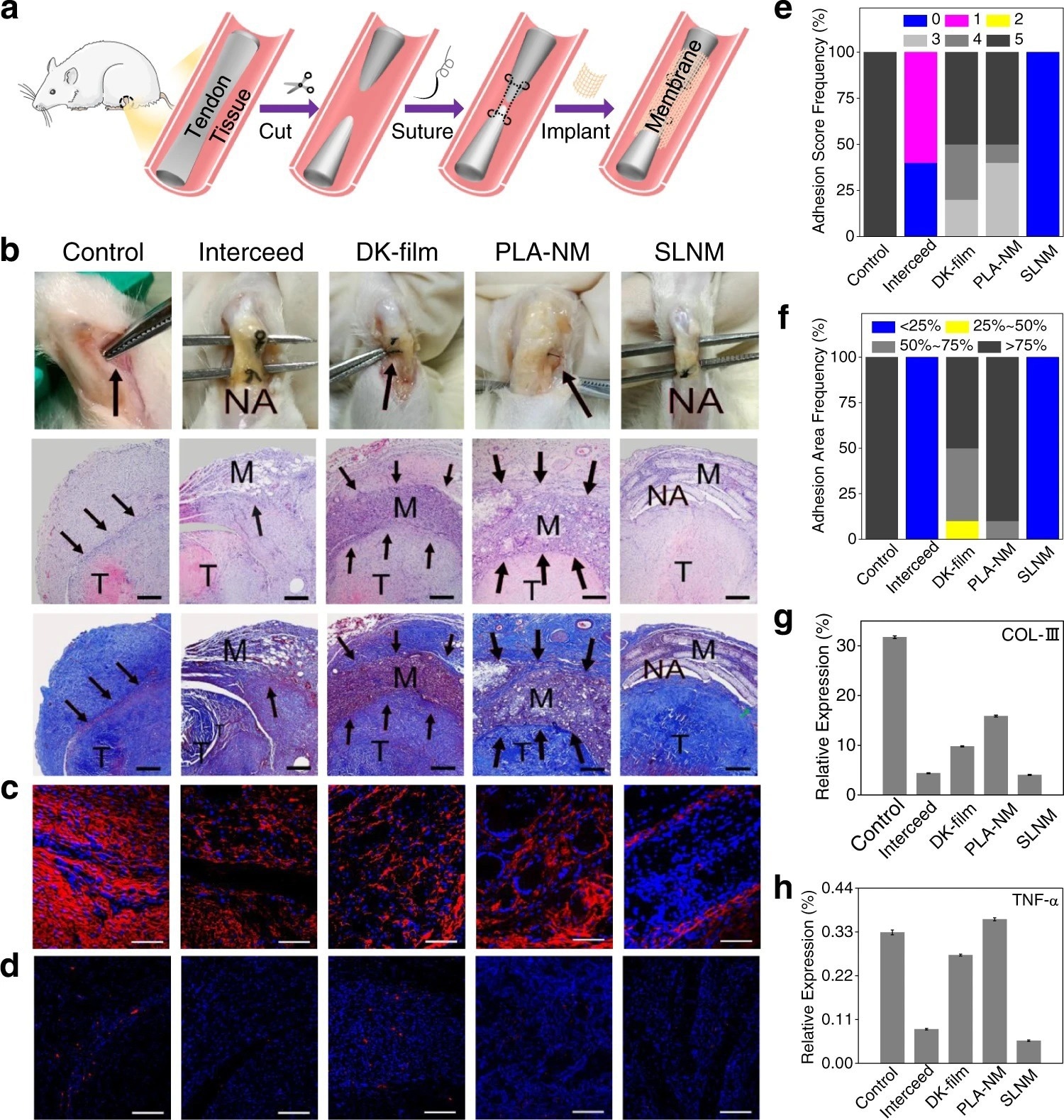
Figure 2. In vivo antitissue adhesion properties of the superlubricated electrospun nanofibrous membranes based on rat tendon adhesion model. a Schematic diagram showing the overall animal test process. b Photos of the harvested tendon on 14 d following implantation and H&E staining as well as Masson staining images. Scale bar: 500 µm. The black arrows point to adhesion site. M membrane. T tendon. NA no adhesion. Representative confocal laser scanning microscopic images for the immunofluorescent staining of c COL-III (scale bar: 100 µm) and d TNF-α (scale bar: 200 µm). Red color represents targeted protein and blue color represents cell nucleus. Comparison of e Adhesion score, f Adhesion area, and relative expression levels of g COL-III as well as h TNF-α for the control, Interceed, DK-film, PLA-NM, and SLNM groups, respectively. The experiments in b–d are replicated three times independently with similar results. © Wang, Y., Xu, Y., Zhai, W. et al. (2022)
Development of Superlubricated Nano-skin on Electrospun Nanofibers
In a previous method, after soaking in benzophenone, the electrospun nanofibrous membranes were immersed in an aqueous solution of methacryloyloxyethyl phosphorylcholine (MPC) monomer, which is a hydrophilic initiator 2-hydroxy-1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-1-propanone (I-2959). The next step was to expose to ultraviolet light, for 30 minutes, on each side. The membrane was rinsed with enough deionized water to remove the weakly linked PMPC molecules. The newly synthesized surface-functionalized electrospun PLA nanofibers containing PMPC on the surface exhibited hydration lubrication performance.
Recently, in situ superlubricated nano-skin (SLNS) was grown (inside-out) on an electrospun nanofiber surface. During the electrospinning process, I-2959 (hydrophilic small molecules) self-arranged in the subsurface of the PLA nanofibers (hydrophobic polymer). The main advantage of this technique is that the second initiator outside the nanofibers is not required. As stated above, photopolymerization was performed by subjecting both sides of the electrospun PLA/I-2959 nanofibrous membranes to ultraviolet rays for 30 minutes, which was then immersed in an aqueous MPC monomer solution. The electrospun nanofiber membrane was rinsed with deionized water to remove unbound MPC and, thereby, superlubricated membranes were synthesized.
Both the techniques described were compared using various analytical tools, such as X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM). The surface elemental compositions of the newly synthesized superlubricated membranes were analyzed using XPS.
The electrospun nanofiber synthesized using the first method showed the presence of a phosphorous element, which originates from PMPC. Hence, XPS data indicated that PMPC coating was successfully prepared in the first method. However, a SEM analysis showed prominent damage to nanofibers, which strongly suggests that hydrogel-skin coating cannot be applied to nanofibers. Nevertheless, SEM and XPS data of the newly developed superlubricated nano-skin (SLNS) showed successful coating without destroying nanofiber structures.
Importantly, the in situ grown superlubricated coating formed on the electrospun nanofiber surface with a thickness of around 1 to 10 nm. Additionally, the COF was lower than 0.025. The newly developed nanofibrous membranes exhibited ideal tensile property and biocompatibility.
Figure 3. Potential antiadhesion mechanism of the superlubricated electrospun nanofibrous membranes. a Schematic diagram showing the occurrence of postoperative tissue adhesion. Interstitial fibrosis and inflammation are involved in this process. Schematic diagrams showing the mechanisms of b inhibiting fibrosis and c reducing inflammation based on the tenacious hydration layer formed surrounding the zwitterionic phosphorylcholine groups on the SLNM surface. © Wang, Y., Xu, Y., Zhai, W. et al. (2022)
Anti-adhesion Performance of Superlubricated Nano-Skin on Electrospun Nanofibers
The newly developed SLNM with the superlubricated nano-skin exhibited significant anti-adhesion performance. Importantly, compared to two commercially used anti-adhesion products, namely, DK-film and intercede, the newly synthesized material showed greater anti-adhesion performance with a lower production cost. This finding was validated using an in vitro anti-cell adhesion test and an in vivo study (anti-tissue adhesion test) was also performed using rat abdominal adhesion and tendon adhesion models. In the future, the application of the newly developed superlubricated biomaterial could prevent post-operative adhesion.
News
Scientists discover cancer-fighting bacteria that ‘soak up’ forever chemicals in the body
A family of healthy bacteria may help 'soak up' toxic forever chemicals in the body, warding off their cancerous effects. Forever chemicals, also known as PFAS (per- and polyfluoroalkyl substances), are toxic chemicals that [...]
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]