The advent of evidence based medicine was a paradigm shift intended to provide a solid scientific foundation for medicine. The validity of this new paradigm, however, depends on reliable data from clinical trials, most of which are conducted by the pharmaceutical industry and reported in the names of senior academics. The release into the public domain of previously confidential pharmaceutical industry documents has given the medical community valuable insight into the degree to which industry sponsored clinical trials are misrepresented.1234 Until this problem is corrected, evidence based medicine will remain an illusion.
The philosophy of critical rationalism, advanced by the philosopher Karl Popper, famously advocated for the integrity of science and its role in an open, democratic society. A science of real integrity would be one in which practitioners are careful not to cling to cherished hypotheses and take seriously the outcome of the most stringent experiments.5 This ideal is, however, threatened by corporations, in which financial interests trump the common good. Medicine is largely dominated by a small number of very large pharmaceutical companies that compete for market share, but are effectively united in their efforts to expanding that market. The short term stimulus to biomedical research because of privatisation has been celebrated by free market champions, but the unintended, long term consequences for medicine have been severe. Scientific progress is thwarted by the ownership of data and knowledge because industry suppresses negative trial results, fails to report adverse events, and does not share raw data with the academic research community. Patients die because of the adverse impact of commercial interests on the research agenda, universities, and regulators.
The pharmaceutical industry’s responsibility to its shareholders means that priority must be given to their hierarchical power structures, product loyalty, and public relations propaganda over scientific integrity. Although universities have always been elite institutions prone to influence through endowments, they have long laid claim to being guardians of truth and the moral conscience of society. But in the face of inadequate government funding, they have adopted a neo-liberal market approach, actively seeking pharmaceutical funding on commercial terms. As a result, university departments become instruments of industry: through company control of the research agenda and ghostwriting of medical journal articles and continuing medical education, academics become agents for the promotion of commercial products.6 When scandals involving industry-academe partnership are exposed in the mainstream media, trust in academic institutions is weakened and the vision of an open society is betrayed.
The corporate university also compromises the concept of academic leadership. Deans who reached their leadership positions by virtue of distinguished contributions to their disciplines have in places been replaced with fundraisers and academic managers, who are forced to demonstrate their profitability or show how they can attract corporate sponsors. In medicine, those who succeed in academia are likely to be key opinion leaders (KOLs in marketing parlance), whose careers can be advanced through the opportunities provided by industry. Potential KOLs are selected based on a complex array of profiling activities carried out by companies, for example, physicians are selected based on their influence on prescribing habits of other physicians.7 KOLs are sought out by industry for this influence and for the prestige that their university affiliation brings to the branding of the company’s products. As well paid members of pharmaceutical advisory boards and speakers’ bureaus, KOLs present results of industry trials at medical conferences and in continuing medical education. Instead of acting as independent, disinterested scientists and critically evaluating a drug’s performance, they become what marketing executives refer to as “product champions.”
Ironically, industry sponsored KOLs appear to enjoy many of the advantages of academic freedom, supported as they are by their universities, the industry, and journal editors for expressing their views, even when those views are incongruent with the real evidence. While universities fail to correct misrepresentations of the science from such collaborations, critics of industry face rejections from journals, legal threats, and the potential destruction of their careers.8 This uneven playing field is exactly what concerned Popper when he wrote about suppression and control of the means of science communication.9 The preservation of institutions designed to further scientific objectivity and impartiality (i.e., public laboratories, independent scientific periodicals and congresses) is entirely at the mercy of political and commercial power; vested interest will always override the rationality of evidence.10
Regulators receive funding from industry and use industry funded and performed trials to approve drugs, without in most cases seeing the raw data. What confidence do we have in a system in which drug companies are permitted to “mark their own homework” rather than having their products tested by independent experts as part of a public regulatory system? Unconcerned governments and captured regulators are unlikely to initiate necessary change to remove research from industry altogether and clean up publishing models that depend on reprint revenue, advertising, and sponsorship revenue.
Our proposals for reforms include: liberation of regulators from drug company funding; taxation imposed on pharmaceutical companies to allow public funding of independent trials; and, perhaps most importantly, anonymised individual patient level trial data posted, along with study protocols, on suitably accessible websites so that third parties, self-nominated or commissioned by health technology agencies, could rigorously evaluate the methodology and trial results. With the necessary changes to trial consent forms, participants could require trialists to make the data freely available. The open and transparent publication of data are in keeping with our moral obligation to trial participants—real people who have been involved in risky treatment and have a right to expect that the results of their participation will be used in keeping with principles of scientific rigour. Industry concerns about privacy and intellectual property rights should not hold sway.
THE JAPANESE JOURNAL OF ANTIBIOTICS 74―1 61( 61 ) http://jja-contents.wdc-jp.com/pdf/JJ…
News
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
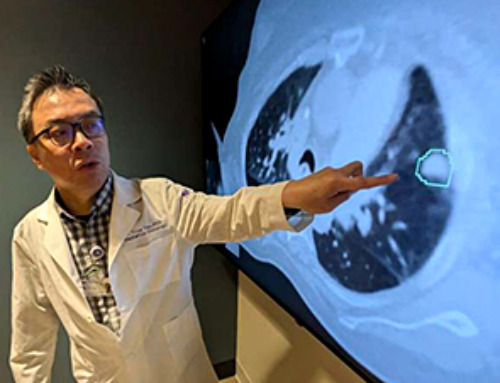
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]
Controlling This One Molecule Could Halt Alzheimer’s in Its Tracks
New research identifies the immune molecule STING as a driver of brain damage in Alzheimer’s. A new approach to Alzheimer’s disease has led to an exciting discovery that could help stop the devastating cognitive decline [...]