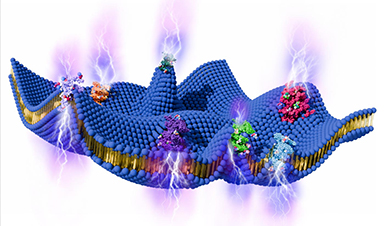
Research led by the University of Pennsylvania, Philadelphia, has used siRNA-based silencing of protein cyclophilin A (CyPA) to reduce tumor burden and extend the lives of patients with multiple myeloma.
Multiple myeloma (MM) is a blood cancer that occurs in the bone marrow and can form tumors outside bone marrow in the body’s organs (extramedullary disease). MM is currently treatable but incurable, with inevitable relapse after treatment and typically short survival rates of 3 to 6 months for those with relapse as they build resistance to the treatments.
Endothelial cells within the bone marrow microenvironment are thought to play a critical role. Specifically, cyclophilin A (CyPA), a protein secreted by bone marrow endothelial cells, is involved in progression, survival, and chemotherapeutic resistance.
Inhibiting CyPA could simultaneously inhibit MM progression and make MM more vulnerable to chemotherapeutics, but getting inhibiting molecules into the bone marrow endothelium is challenging.
Small interfering RNA (siRNA) therapeutics have broad potential to silence any targeted gene and are a great candidate for CyPA silencing but are limited by instability in the bloodstream and an inability to traverse cell membranes easily.
The researchers needed a way to get the potential therapy to the correct location and turned to a nanoparticle delivery system. The team developed nanoparticles comprised of a polymer-lipid hybrid material and a lipid-polyethylene glycol (PEG) to enable nucleic acid encapsulation.
The nanoparticles reduced the degradation of the RNA by enzymes in the blood and were able to deliver the payload siRNA to specific tissues via functionalization of the nanoparticle surface chemistry,
The nanoparticle delivery platform and a CyPA silencing siRNA payload were deployed in a living mouse model, and it worked. The silencing of CyPA decreased multiple myeloma invasion across bone marrow endothelial cells and disrupted interactions. When combined with a therapeutic, bortezomib, CyPA silencing sensitized cancer cells to therapy, which reduced proliferation and angiogenesis, and ultimately extended mouse survival.
The authors suggest that this nanoparticle platform may provide a broadly enabling technology to deliver nucleic acid therapeutics to other malignancies.
News
A Forgotten Molecule Could Revive Failing Antifungal Drugs and Save Millions of Lives
Scientists have uncovered a way to make existing antifungal drugs work again against deadly, drug-resistant fungi. Fungal infections claim millions of lives worldwide each year, and current medical treatments are failing to keep pace. [...]
Scientists Trap Thyme’s Healing Power in Tiny Capsules
A new micro-encapsulation breakthrough could turn thyme’s powerful health benefits into safer, smarter nanodoses. Thyme extract is often praised for its wide range of health benefits, giving it a reputation as a natural medicinal [...]
Scientists Develop Spray-On Powder That Instantly Seals Life-Threatening Wounds
KAIST scientists have created a fast-acting, stable powder hemostat that stops bleeding in one second and could significantly improve survival in combat and emergency medicine. Severe blood loss remains the primary cause of death from [...]
Oceans Are Struggling To Absorb Carbon As Microplastics Flood Their Waters
New research points to an unexpected way plastic pollution may be influencing Earth’s climate system. A recent study suggests that microscopic plastic pollution is reducing the ocean’s capacity to take in carbon dioxide, a [...]
Molecular Manufacturing: The Future of Nanomedicine – New book from Frank Boehm
This book explores the revolutionary potential of atomically precise manufacturing technologies to transform global healthcare, as well as practically every other sector across society. This forward-thinking volume examines how envisaged Factory@Home systems might enable the cost-effective [...]
New Book! NanoMedical Brain/Cloud Interface – Explorations and Implications
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
Global Health Care Equivalency in the Age of Nanotechnology, Nanomedicine and Artificial Intelligence
A new book by Frank Boehm, NanoappsMedical Inc. Founder. This groundbreaking volume explores the vision of a Global Health Care Equivalency (GHCE) system powered by artificial intelligence and quantum computing technologies, operating on secure [...]
Miller School Researchers Pioneer Nanovanilloid-Based Brain Cooling for Traumatic Injury
A multidisciplinary team at the University of Miami Miller School of Medicine has developed a breakthrough nanodrug platform that may prove beneficial for rapid, targeted therapeutic hypothermia after traumatic brain injury (TBI). Their work, published in ACS [...]
COVID-19 still claims more than 100,000 US lives each year
Centers for Disease Control and Prevention researchers report national estimates of 43.6 million COVID-19-associated illnesses and 101,300 deaths in the US during October 2022 to September 2023, plus 33.0 million illnesses and 100,800 deaths [...]
Nanomedicine in 2026: Experts Predict the Year Ahead
Progress in nanomedicine is almost as fast as the science is small. Over the last year, we've seen an abundance of headlines covering medical R&D at the nanoscale: polymer-coated nanoparticles targeting ovarian cancer, Albumin recruiting nanoparticles for [...]
Lipid nanoparticles could unlock access for millions of autoimmune patients
Capstan Therapeutics scientists demonstrate that lipid nanoparticles can engineer CAR T cells within the body without laboratory cell manufacturing and ex vivo expansion. The method using targeted lipid nanoparticles (tLNPs) is designed to deliver [...]
The Brain’s Strange Way of Computing Could Explain Consciousness
Consciousness may emerge not from code, but from the way living brains physically compute. Discussions about consciousness often stall between two deeply rooted viewpoints. One is computational functionalism, which holds that cognition can be [...]
First breathing ‘lung-on-chip’ developed using genetically identical cells
Researchers at the Francis Crick Institute and AlveoliX have developed the first human lung-on-chip model using stem cells taken from only one person. These chips simulate breathing motions and lung disease in an individual, [...]
Cell Membranes May Act Like Tiny Power Generators
Living cells may generate electricity through the natural motion of their membranes. These fast electrical signals could play a role in how cells communicate and sense their surroundings. Scientists have proposed a new theoretical [...]
This Viral RNA Structure Could Lead to a Universal Antiviral Drug
Researchers identify a shared RNA-protein interaction that could lead to broad-spectrum antiviral treatments for enteroviruses. A new study from the University of Maryland, Baltimore County (UMBC), published in Nature Communications, explains how enteroviruses begin reproducing [...]
New study suggests a way to rejuvenate the immune system
Stimulating the liver to produce some of the signals of the thymus can reverse age-related declines in T-cell populations and enhance response to vaccination. As people age, their immune system function declines. T cell [...]