A novel freeze-dissolving approach has been devised that offers greater efficiency and sustainability compared to the classic freeze-drying process to make superfine powder or nanoparticles.
In the research published in the journal ACS Sustainable Chemistry & Engineering, sphere-shaped ice particles were formed in an aqueous mixture of NH4H2PO4 or NaHCO3 to produce their respective nanoparticles.
What is the Freeze-Drying Method?
Due to their significant specific areas and strong reactivity, nanomaterials and superfine powders are gaining popularity in fields such as sustainable and environmental applications.
Nanoparticles (NPs) and superfine powders are often produced using freeze-drying techniques. The initial stage in the freeze-drying technique is a cryogenic procedure that freezes target particles or molecules in an aqueous mixture.
In the aqueous mixture, water molecules solidify quickly via the fast-freeze stage, generating a framework of crystallized ice. This step is also referred to as ice templating or freeze-casting. The crystallized ice framework forces the targeted dissolved molecules or components to produce a nanoscale scaffolding architecture, which results in substances with nanoscale or microscale pores.
The freezing stage defines the architecture of the scaffolding and the ice template, as well as the crystal architecture of the targeted substances inside the ice templates or scaffolds, based on the freezing settings.
The second phase is a drying procedure that uses the process of sublimation to separate water as ice templates. The ice melts throughout the drying phase, but the targeted substances, particles, or molecules stay within the ice. From inside the ice, freeze-cast NPs or porous substances with identical architecture and characteristics may be retrieved.
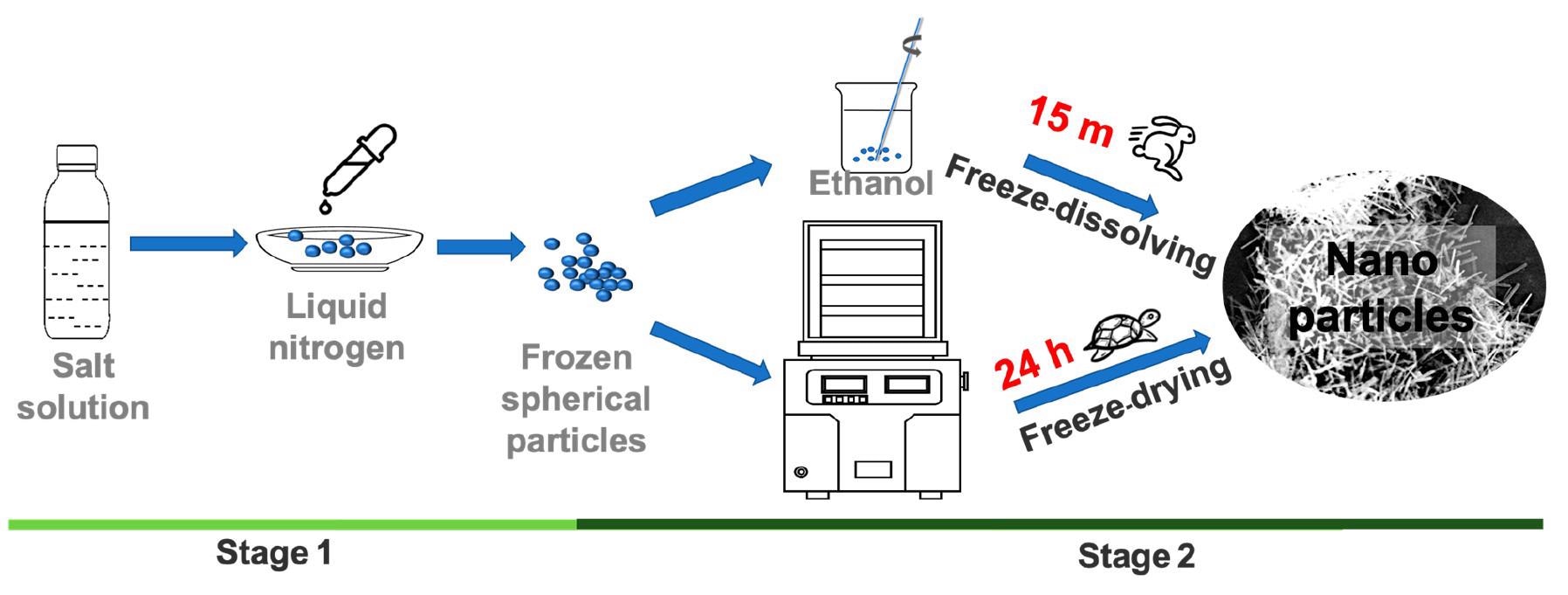
Schematic diagram of experimental setup for the freeze-dissolving method (top) and the freeze-drying method (bottom). © Yu, Q., Wang, Y., Luo, J., & Yang, H. (2022).
Limitations of Freeze-Drying
Due to the cooler temperatures employed in the drying phase, sublimation speeds are sluggish, and batch drying periods for common pharmacological items can take up to multiple days. The production speeds of such batch-based technologies are constrained by poor freeze-drying speeds and extended cycle operation durations.
Some drawbacks may be mitigated by purchasing a bigger freeze-dryer. Unfortunately, it takes much more time to establish perfect vacuum settings, and temperature and pressure are less consistent throughout the container, which could influence output quality. As a result of the cold temperatures and the vacuum arrangement, the drying phase consumes a lot of energy.
How is the Freeze-Dissolving Method Better?
The initial stage in freeze-dissolving is identical to that of freeze-drying, that is, freeze-casting to create ice containing the target components within and build an ice scaffold target architecture.
The ice is then dissolved at a cold temperature, such as a sub-zero temperature in an additional solvent having a low freezing point in the subsequent phase of the freeze-dissolving process. This additional solvent, like ethanol, acts as an antisolvent for the targeted components yet shows miscibility with water.
As a result, the ice scaffold will dissipate fast in the additional solvent, leaving just the targeted components in a solid-state in the mixture, and the architecture of the targeted components produced within the ice will be conserved.
Fire suppression chemicals, baking soda, ammonium dihydrogen phosphate (NH4H2PO4), and sodium bicarbonate (NaHCO3) are water-soluble but do not dissolve in ethanol.
In this work, various quantities of sodium bicarbonate or ammonium dihydrogen phosphate, dissolved in water, were employed to manufacture NPs via the freeze-dissolving technique, which were then evaluated against NPs produced by freeze-drying.
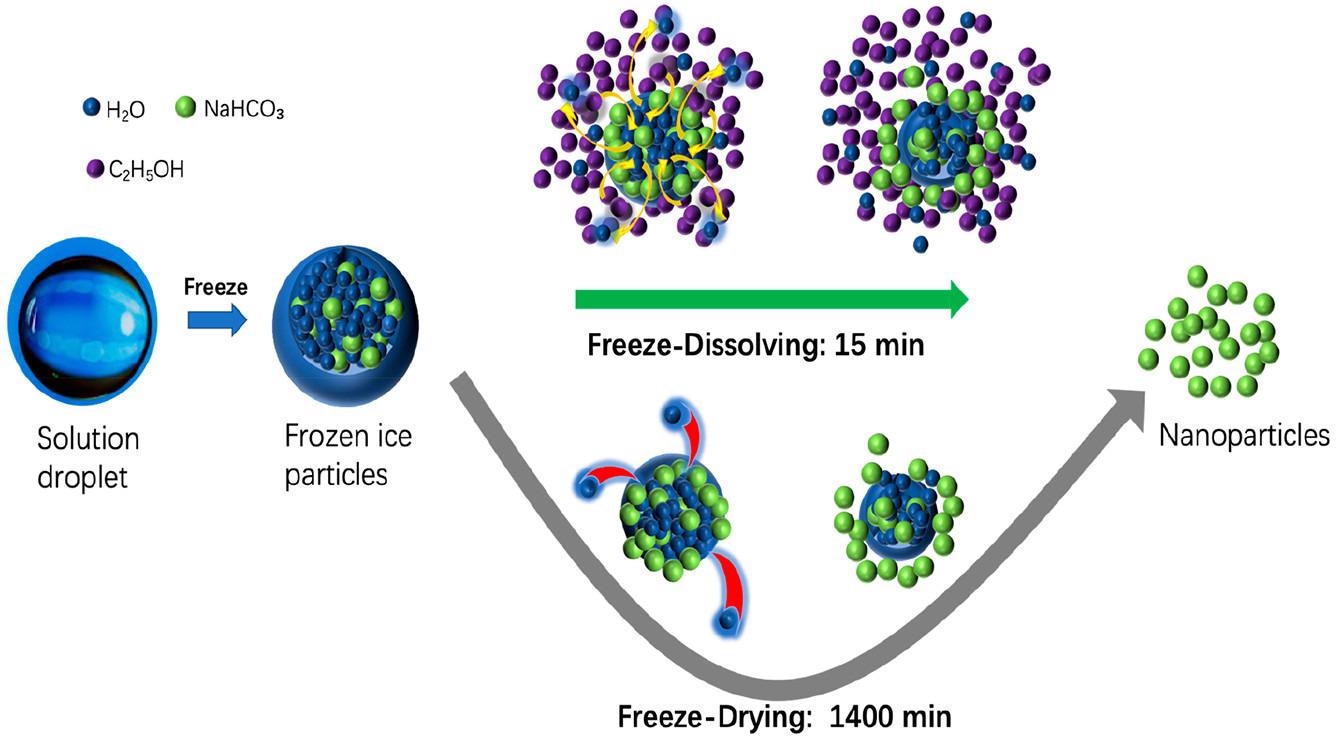
Schematic diagram of the freeze-dissolving and freeze-drying mechanisms for the formation and isolation of NaHCO3 nanoparticles. © Yu, Q., Wang, Y., Luo, J., & Yang, H. (2022).
Important Findings
To extract superfine powder and NPs from ice templates within frozen particles, the proposed freeze-dissolving process offers greater efficiency and sustainability compared to the conventional freeze-drying approach.
Particles of sodium bicarbonate and ammonium dihydrogen phosphate aqueous mixtures were quickly frozen to produce sphere-shaped ice particles, which were then filled with NPs and superfine powder of NaHCO3 or NH4H2PO4.
The frozen components were dispersed in ethanol for 5 minutes at 10 °C using the freeze-dissolving procedure to separate the ice scaffold. The freeze-drying approach, on the other hand, needed 1400 minutes to separate the ice scaffold via the process of sublimation. In identical experimental settings, the dimensions of the end products generated by the freeze-dissolving approach were comparatively small as opposed to those produced by the freeze-drying approach.
The freezing-dissolving approach reported in this study is approximately 100 times quicker and consumes roughly 100 times lesser energy as compared to the freeze-drying approach, without the need for a large facility or a vacuum. As a result, the freeze-dissolving process is likely to be used on an industrial scale with less time, energy, and footprint.
News
Johns Hopkins Researchers Uncover a New Way To Kill Cancer Cells
A new study reveals that blocking ribosomal RNA production rewires cancer cell behavior and could help treat genetically unstable tumors. Researchers at the Johns Hopkins Kimmel Cancer Center and the Department of Radiation Oncology and Molecular [...]
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]
Controlling This One Molecule Could Halt Alzheimer’s in Its Tracks
New research identifies the immune molecule STING as a driver of brain damage in Alzheimer’s. A new approach to Alzheimer’s disease has led to an exciting discovery that could help stop the devastating cognitive decline [...]