Researchers want to know why cells produce tiny packages called vesicles — and whether these bundles could be used for therapy.
Graça Raposo was a young postdoc in the Netherlands in 1996 when she discovered that cells in her laboratory were sending secret messages to each other.
She was exploring how immune cells react to foreign molecules. Using electron microscopy, she saw how cells ingested these molecules, which became stuck to the surface of tiny intracellular vesicles. The cells then spat out the vesicles, along with the foreign cargo, and Raposo captured them.
Next, she presented them to another type of immune cell. It reacted to the package just as it would to a foreign molecule1.
It was a demonstration that these extracellular vesicles (EVs, also known as exosomes) might be transmitting information between cells. “We knew that exosomes existed, but at that time they were generally thought to be a way of getting rid of a cell’s trash,” says Raposo. “It was exciting to find that some could have important biological functions — even if not everyone believed it at first.”
Just two years later, together with her colleagues, Raposo, who is now at the Curie Institute in Paris, found that exosomes derived from antitumour immune cells could be enlisted to suppress cancers in mice2.
Other scientists jumped on the concept, and began to find exosomes being spat out from all kinds of cell. Interest exploded after researchers discovered that the little packages could contain not only proteins, but also nucleic acids. In 2007, for instance, a Swedish team found that the vesicles harboured small RNA molecules3. That implied that exosomes might influence gene expression when they reached their destination cells. The number of EV-related publications rose steeply, and has tripled in the past five years.
The shape-shifting blobs that shook up cell biology
As the field has matured, researchers have set out more-stringent rules for what counts as an EV and have established guidelines for how to identify these minuscule bubbles in living systems. Scientists are learning ever more about how vesicles move in and out of cells and across the complex terrain between them, as well as about the roles of vesicles in health and disease. EVs might, for example, be responsible for cancers spreading in the body, or could even be involved in the ageing process.
It is all very much a work in progress, but there is a strong sense of promise in the field. Researchers hope that EVs could be exploited to diagnose, or even treat, various diseases. The number of patent applications and registered clinical trials has soared in parallel with the rise in publications.
But there is also a dark side. To the concern of regulatory agencies, hundreds of private clinics and companies selling unproven EV-based therapies have sprung up around the world, offering to treat anything from Alzheimer’s disease to baldness. “The number of direct-to-consumer companies peddling EV treatments without proof of safety or efficacy is shocking,” says stem-cell biologist Darius Widera at the University of Reading, UK.
Tiny but mighty
It can be difficult enough to see whole cells under a microscope, let alone detect the nanoscale particles that they eject. Scientists who have embraced this challenge have defined around half a dozen broad classes of EVs according to the way in which they are created and expelled.
The classes have overlapping sizes. Exosomes, with a size range of 30–150 nanometres, are the smallest class. They are produced by machinery inside the cell that traffics molecules and particles to where they need to be. Exosomes are held in larger packages, which move to the cell membrane, fuse with it and release the vesicles to the outside. Another class, known as ectosomes, has the widest size range: 50–10,000 nm. Ectosomes pinch off from the edge of the main cell membrane (see ‘Special delivery’).
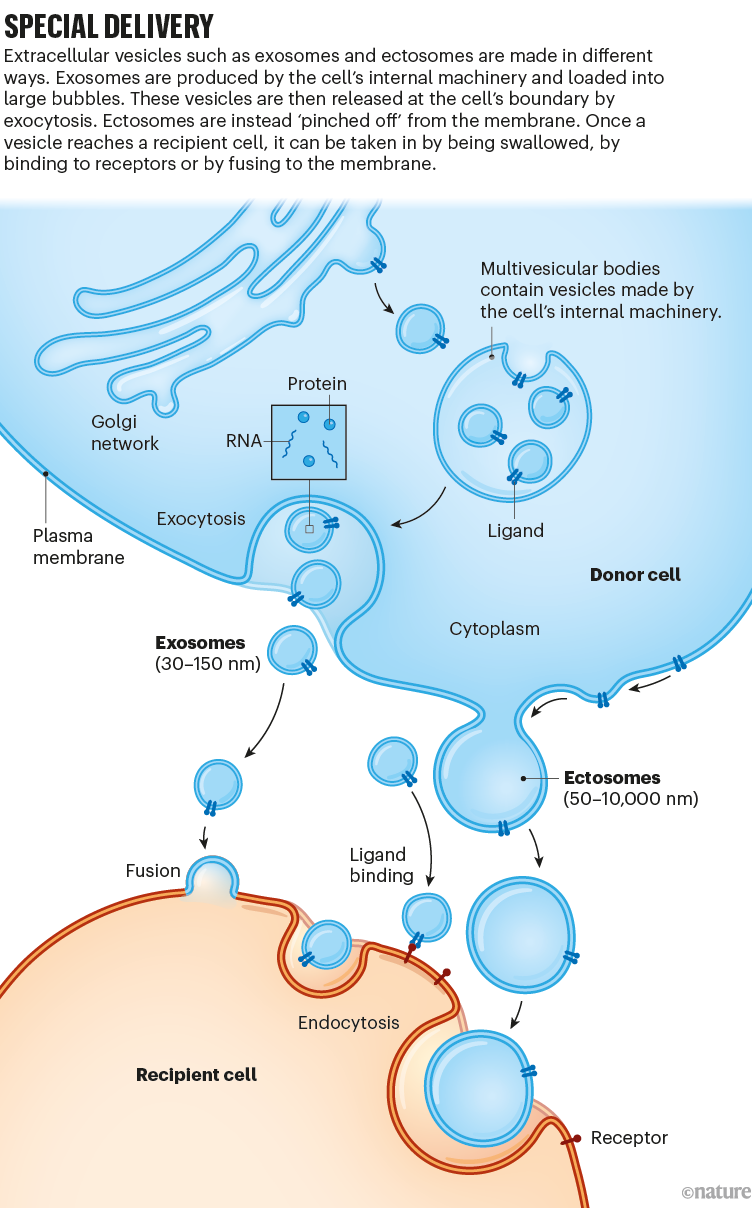
In these classes, hundreds, if not thousands, of types of EV carrying a broad range of cargo might exist, created by pathways in different cells at various times and in different states of health. Nearly all types of cell (including those from plants, fungi and bacteria) generate a rich variety of vesicles. Many types overlap in terms of size and the repertoire of marker molecules on their surfaces, so they are hard to differentiate.
What all these vesicles are doing is a big open question. Many simply carry out the cell’s rubbish — by-products and toxins, for instance — and send some of it to the liver for breakdown. Others communicate with cells in the same or different tissues. Some vesicles do this through signalling molecules on the cells’ surface (such as the EVs from Raposo’s immune cells); others do so by entering cells and delivering their cargo internally. Yet others could be involved in indirect communication, for example, by releasing their contents outside cells to create a supportive environment for themselves.
EVs released from healthy cells are known to regulate a wide range of physiological processes, from triggering an immune response to maintaining cellular equilibrium. Those from diseased cells might help to spread the disease. EVs from tumour cells, for example, carry cancer-promoting proteins and RNA molecules to other sites in the body, where they can develop as metastases. In neurodegenerative diseases, such as Alzheimer’s, that are associated with misfolded proteins, including amyloid-β, tau and α-synuclein, scientists suspect that EVs secreted by neurons might be responsible for spreading these proteins — and therefore the disease — across the brain.
These discoveries have been hard-won owing to the technical challenges of studying such tiny and extraordinarily varied particles. In fact, much of the early work in the field has proved difficult to interpret because of confusion related to both nomenclature and claims. “Until recently it was like the Wild West,” says Rienk Nieuwland at the Amsterdam University Medical Centers. Measurements of EVs could vary by several orders of magnitude, even in standard blood samples, he says, and some scientists worried that the uncertainties threatened to undermine the whole field.
Over the past three years, the International Society for Extracellular Vesicles in Mount Royal, New Jersey, has tried to bring order to the field by recommending standardized methods for preparing EVs and for naming them. Nieuwland says that measurements have become much less variable between labs.
Researchers still have a lot of questions about the biology of EVs. For example, how exactly do vesicles find their way to their target cells? And once inside, does their cargo function as assumed? Perhaps the biggest test for the field is whether EVs behave in the same way in the body as they do in cell culture. To know for certain, scientists would have to see how these tiny vesicles act in a whole organism. “I’ve never seen conclusive evidence that EVs do anything in vivo,” says Nieuwland.
Delivery service
Still, researchers say that enough is known about EVs to justify explorations of some areas of clinical potential. Scientists have realized that these cargo carriers could serve as Trojan horses to enter particular cells and change them in useful ways — for example, by targeting cancer cells for destruction, or delivering drugs or gene therapy.
Jan Lötvall at the University of Gothenburg in Sweden inspired many drug developers when his team found that exosomes could transport RNA3. The researchers showed that the exosomes could pass from one immune cell to another and that their contents could be translated into biologically active proteins, at least in vitro. (Whether cargo RNA is capable of being translated in recipient cells in vivo is the subject of much debate.)
Matthew Wood at the University of Oxford, UK, immediately saw the potential for delivering therapy to cells. He had been trying to develop treatments for inherited genetic disorders such as Duchenne muscular dystrophy for more than a decade when he read Lötvall’s paper.
He and others thought EVs could be used in the emerging field of gene therapy, which was showing promise for replacing or modifying defective genes. “But the challenge was always to deliver the genetic therapy to the right cells in the right tissue,” says Wood. Muscular dystrophy, for example, affects all muscles in the body, including the heart, and also affects the brain, so genetic abnormalities would need to be corrected in these places without disturbing cells in other, healthy tissues. That’s a big ask, says Wood.
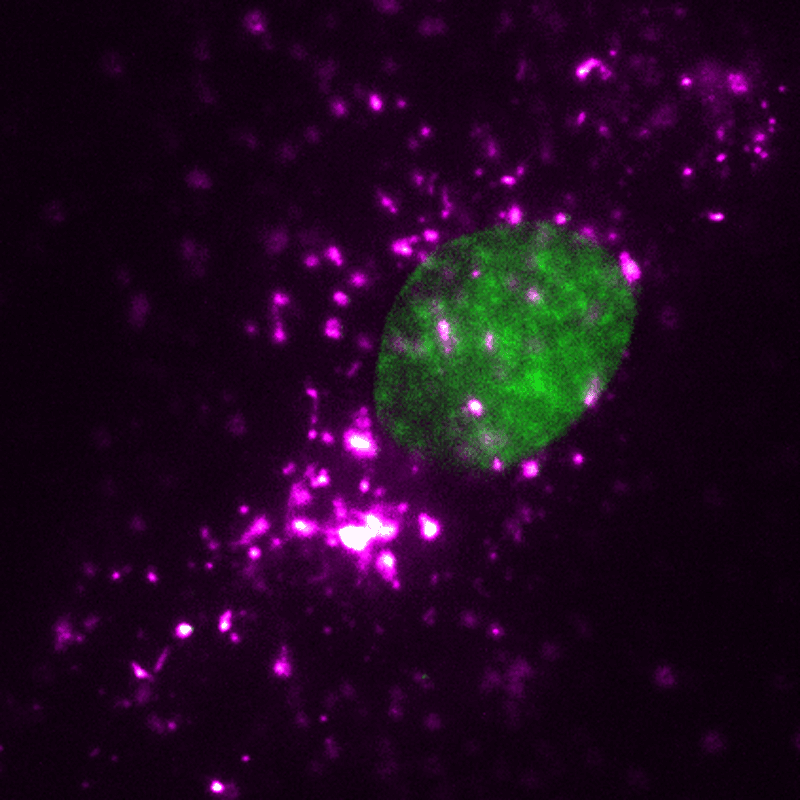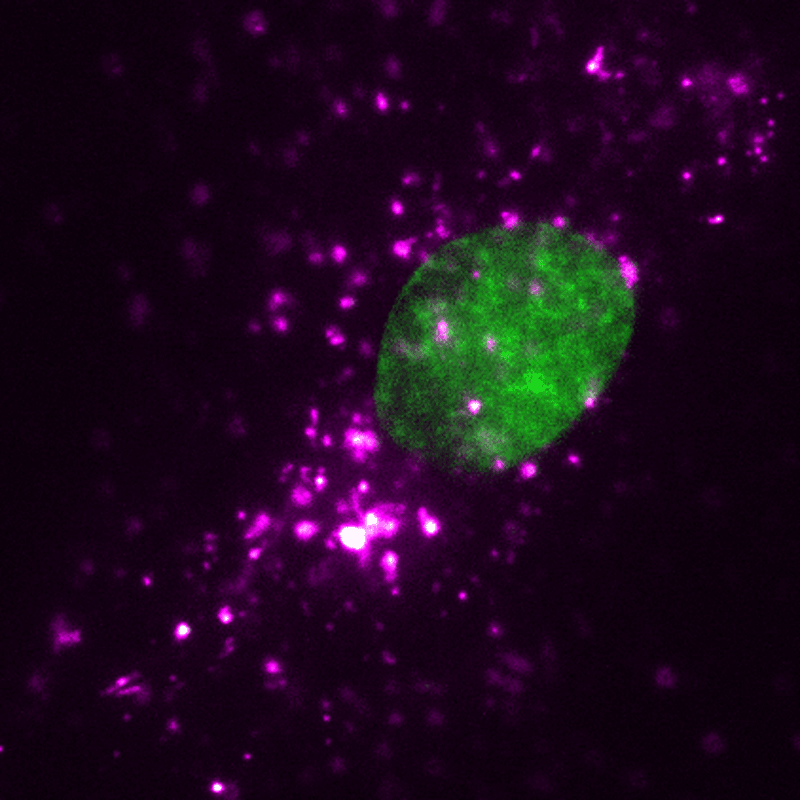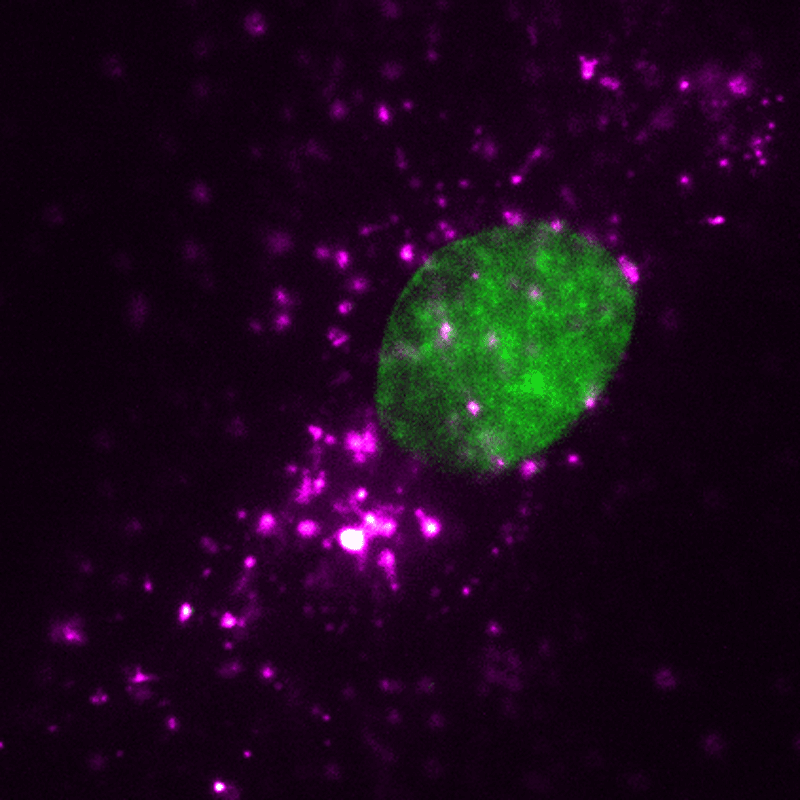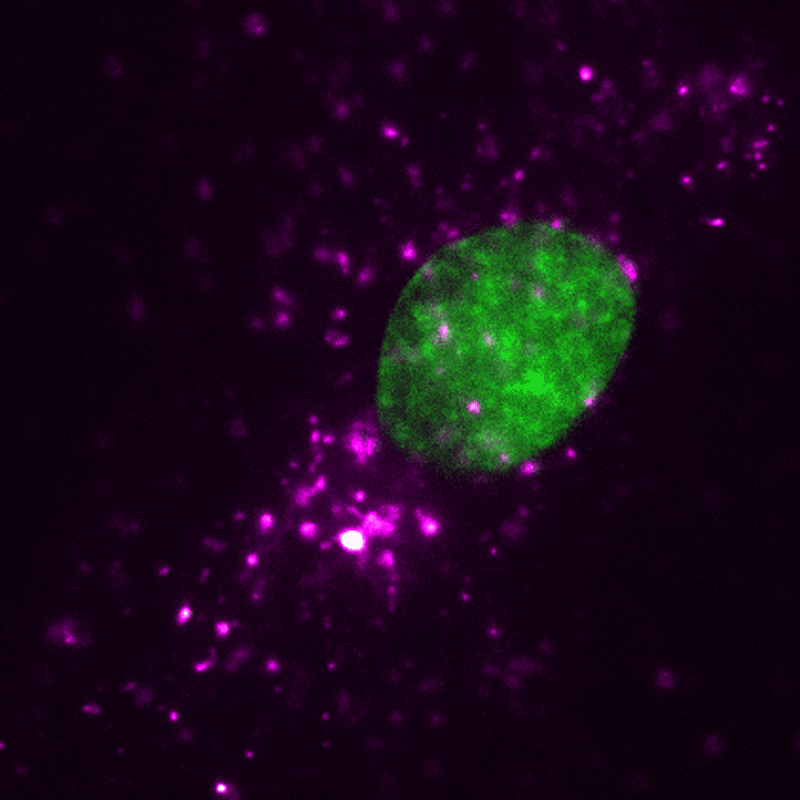
EVs (magenta) can be tracked in a living human cell (nucleus shown in green) using super-resolution microscopy. Credit: ONI (Oxford Nanoimaging)/Christopher Gregory (University of Edinburgh Centre for Inflammation Research)
One way researchers were getting genes to cells was inside synthetic nanoparticles — the same method now used in messenger-RNA vaccines for COVID-19. Wood wondered whether naturally produced EVs might have the potential to make therapies better tolerated than synthetic particles, which can cause inflammation. He was convinced that, if appropriately engineered, they could make excellent gene-therapy vectors.
It took him four years to prove the principle that EVs could indeed be designed to deliver functional genetic material to target cells. For this test, he worked on the misfolded proteins of Alzheimer’s disease. He isolated EVs from a type of immune cell in mice, designed them to target neurons and got them to load up a small RNA that switches off a gene for an enzyme involved in the disease. When he injected the vesicles into mice, levels of the enzyme dropped by two-thirds4.
In 2021, he and his colleagues made EVs that can carry and deliver useful proteins, and used them to suppress inflammation that contributes to skeletal muscle weakness in a mouse model of Duchenne muscular dystrophy5.
Many companies, including Oxford-based Evox Therapeutics, co-founded by Wood, are now developing EV systems to deliver gene therapy for various diseases. The technology has become ever more sophisticated — EVs can be made to carry various molecules that allow a tissue to express new genes, silence them or even edit genes. So far, no gene therapy using EVs has been registered for a clinical trial.
Some researchers are looking at whether EVs can deliver other types of molecule. Scientists in China are carrying out early-stage clinical trials in which EVs derived from a person’s own tumour cells are loaded with a chemotherapeutic agent, such as methotrexate or cisplatin6. And small trials using engineered EVs to treat some cancers are ongoing7.
Some treatments require a specific type of EV. But for therapies that require EVs simply as a vessel, most researchers make them from a type of stem cell called a mesenchymal stem cell. That’s because these cells have been tested, safely and often successfully, in hundreds of clinical trials for cardiovascular, neurological, autoimmune and other disorders — although none has yet been approved by the US Food and Drug Administration (FDA).
Scientists originally thought that the healing power of mesenchymal stem cells lay in their potential to differentiate into replacement cell types. But in 2010, Sai Kiang Lim at the A*STAR Institute of Molecular and Cell Biology in Singapore and her colleagues showed8 that the cells’ desirable properties were conveyed by a class of particularly small EVs that they secreted. The focus of many clinical trials moved from the cells themselves to their EVs.
EVs have important advantages over using whole stem cells for therapy. Cells can replicate, and carry a small risk of engrafting themselves or of differentiating into cells that could cause immune reactions. EVs avoid these risks and are easier and cheaper to manufacture, store and transport. They are being tested in early-phase clinical trials for a range of disorders in which inflammation plays a part in worsening the condition, such as for stroke or COVID-19.
Their potential in many other conditions is also being actively studied in animal models, including in myocardial infarction and skin disorders. They might even have anti-ageing potential. When old mice were treated with EVs secreted from mesenchymal stem cells derived from the fat of young mice, they lived longer, were less frail and had more youthful biochemical profiles than control animals did9.
Useful markers
As well as potential treatments, EVs could serve as telltale indicators of disease. Different EVs, including those from diseased cells, tend to have their own signature combination of molecules such as proteins, RNAs and lipids that can serve as biomarkers. Many companies are developing EV-based diagnostic tests for various disorders, particularly those in which early detection can improve prognosis.
In 2019, the FDA granted access to an accelerated approval process for an EV-based diagnostic test for the first time — a urine test for prostate cancer made by biotechnology firm Exosome Diagnostics in Waltham, Massachusetts. Another company, Exosome Sciences in San Diego, California, is developing a test of blood plasma known as TauSome. This detects tau protein that is associated with EVs to diagnose chronic traumatic encephalopathy, a neurodegenerative brain disorder that results from repetitive head trauma. In a preliminary clinical study10, the test identified higher tau levels in former US National Football League players compared with athletes who had participated in non-contact sports.
EVs have another feature that makes them attractive for diagnosis. Because exosomes often contain genetic material, “that opens the exciting prospect of developing very sensitive diagnostic tests”, says neuroscientist Andrew Hill at Victoria University in Melbourne, Australia, who is working on tests to detect neurodegenerative diseases, including Alzheimer’s, based on levels of microRNAs in EVs in the blood. Aside from the diagnostic potential, these tests could also help researchers to understand more about the pathogenic mechanisms in these diseases, they say.
Questionable clinics
A shadier market has already developed in parallel, however. Clinics are sprouting up around the world offering EV treatments for various conditions, from the cosmetic to the life-threatening. A 2023 analysis11 found that around 110 companies market such treatments, several of which operate clinics in multiple countries. “This is all happening without any evidence of safety or efficacy,” says Widera, a co-author of the paper. “The whole grey-zone area is poorly regulated.” The International Society for Extracellular Vesicles has warned about the risk of such unproven therapies, which it says could be contaminated or simply ineffective and a waste of money.
“My worry is not that the EVs could be intrinsically bad,” says Lim. “The problem is that no one checks their production — it is very easy to introduce contaminants, like endotoxin or bacteria.” Indeed, in 2019, the FDA put out a statement warning of the dangers after several people in Nebraska had serious adverse side effects, including sepsis, following treatment with exosomes. The FDA said that clinics that offer unregulated products “deceive patients with unsubstantiated claims about the potential for these products to prevent, treat or cure various diseases or conditions”. Claims by many clinics that they do not need to be regulated are “simply untrue”, the agency says.
Despite concerns about EV clinics, scientists who are interested in the basics of these carriers are forging ahead. They are trying to work out exactly how and where vesicles deliver their cargo, and how to exploit their potential to distribute messages and cellular components. The progress from first discoveries to attempted medical applications has been astonishingly quick, says Raposo. “But there is still such a lot we have to understand.”
Nature 621, 462-464 (2023)
News
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]
Controlling This One Molecule Could Halt Alzheimer’s in Its Tracks
New research identifies the immune molecule STING as a driver of brain damage in Alzheimer’s. A new approach to Alzheimer’s disease has led to an exciting discovery that could help stop the devastating cognitive decline [...]
Cyborg tadpoles are helping us learn how brain development starts
How does our brain, which is capable of generating complex thoughts, actions and even self-reflection, grow out of essentially nothing? An experiment in tadpoles, in which an electronic implant was incorporated into a precursor [...]