Scientists are closing in on biomarkers that reflect the progression of Alzheimer’s disease and could improve treatments.
Like many Alzheimer’s researchers, neurologist Randall Bateman is not prone to effusiveness, having endured disappointments in his field. But he and others have found one big reason to be excited lately. In just a few years, he predicts, there will be a simple blood test for your risk of Alzheimer’s. “Any family doctor will be able to do it.”
Bateman, who is at Washington University in St. Louis, Missouri, has been running clinical trials related to Alzheimer’s disease for nearly 20 years. “From all I’ve seen, this is a very likely scenario,” he says. “It’ll be just like going to get your blood cholesterol checked and then being given statins if levels are too high.”

Could drugs prevent Alzheimer’s? These trials aim to find out
This extraordinary turnaround in outlook for the disease that affects more than 55 million people worldwide comes down to two things — both of which were thought by many to be nigh on impossible just a decade ago. First, drugs that can slow the disease, if it is caught early enough, are now coming on the market. And second, scientists have developed relatively cheap and highly accurate blood-based biomarkers for Alzheimer’s.
These biomarkers — a catch-all term for any biological molecule found in blood or tissue that can indicate someone’s medical state — are not treatments. But they are revolutionizing prospects for therapies that might delay or even prevent Alzheimer’s. They would do this by catching the disease before symptoms — and brain damage — begin.
That hopeful scenario depends on the further development of drugs that can treat or hold off the disease, when caught early. But even now, biomarkers are already improving clinical trials, allowing researchers to test interventions at much earlier stages than before. And they are transforming how researchers track the course of the disease and learn more about its basic pathology. “The pace of development of these tests is extraordinary,” says neurologist Jonathan Schott at University College London. “There is huge excitement.”
Markers of success
Alzheimer’s disease accounts for around two-thirds of all cases of dementia. The brains of people with Alzheimer’s disease have three main characteristics. There are gaps where the tissue has degenerated. The tissue is dotted with plaques — knots of sticky amyloid-β proteins surrounded by immune cells called microglia — and it is laced with stringy tangles formed from tau proteins.
Thanks to the development of biomarkers for both amyloid and tau proteins, scientists have been able to work out the general sequence of the pathology. Plaques develop first, then tangles of tau — and then symptoms. The severity of the symptoms correlates with the extent of tau tangles. The process is so extremely slow that symptoms begin only 10 to 20 years after plaques start to develop.
The idea that defective amyloid proteins could be the drivers of Alzheimer’s disease gained traction in the 1990s, when scientists discovered families with inherited early-onset disease who had mutations in genes involved in amyloid metabolism1. Dozens of clinical trials of drugs targeting amyloid were launched with great fanfare. When they all failed, some scientists started to question the amyloid hypothesis.
But the drugs themselves might not have been the problem. They were being given to the wrong people, or too late. In these early trials, researchers had no good way of selecting participants, choosing appropriate doses or precisely tracking the effects of treatments. “Back then, without biomarkers, we were working blind,” says neurologist Paul Aisen at the University of Southern California, San Diego, who is a leader of the US Alzheimer’s Clinical Trials Consortium.
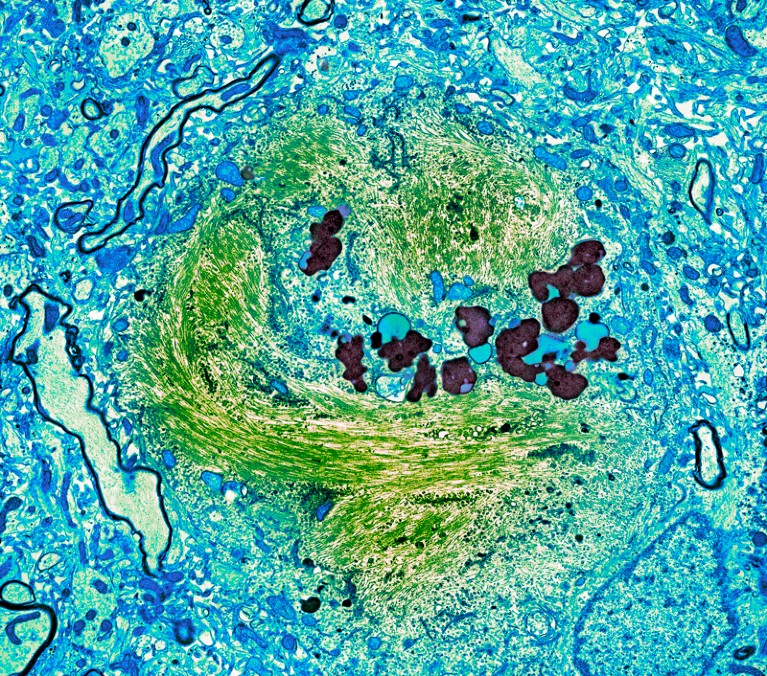
A tau-protein tangle (green) in a neuron from a person with Alzheimer’s disease.Credit: Thomas Deerinck, NCMIR/Science Photo Library
There are several places that Alzheimer’s disease biomarkers can be found: in the brain, in the cerebrospinal fluid (CSF) that bathes the brain and spinal cord and in the blood. Until a couple of years ago, scanning the brain with positron-emission tomography (PET), which allows plaques to be directly visualized and quantified, has been the gold standard, closely corresponding with the pathology seen under the microscope at autopsy.
When amyloid-PET brain scanning became available in 2004, “that made a huge difference”, says Aisen. Researchers were able to use it to study2 the effects of an antibody, aducanumab, that aimed to suppress amyloid. “It showed for the first time that plaques could indeed be removed.”
The aducanumab study, published in 2016, did not show clear clinical benefit, and the drug turned out to cause micro-haemorrhages in some people. But, safer anti-amyloid antibodies have since been developed and tested in clinical trials. Some of the trials have shown that removing plaques, if carried out early enough, can significantly slow disease progression.
Another type of PET scan, this time visualizing tau, was introduced in the mid-2010s. With these two brain-scanning options, the amount and location of both types of Alzheimer’s pathology — plaques and tangles — could now be seen in living people and monitored over time3.
But PET scans are extremely expensive and time-consuming, and they can be performed only in specialized clinics. In the first clinical trial of an anti-amyloid antibody in people with brain plaques but without symptoms, the A4 trial4, it cost around US$40 million to scan 5,000 candidates and select the 1,169 people who would eventually participate.
Conquering Alzheimer’s: a look at the therapies of the future
Instead of looking at the brain itself using PET scans, clinicians can monitor the CSF for two telltale fragments of the amyloid protein, Aβ40 and Aβ42, and various tau peptides. These CSF markers are now almost as accurate as using PET scans and have been included in the US Food and Drug Administration’s guidelines for diagnostics since 2022. But monitoring CSF also has limitations because the fluid has to be acquired by lumbar puncture, an uncomfortable procedure that has to be performed by specialist personnel.
To avoid these complications, scientists have long sought to develop blood tests that would target the same protein biomarkers as the CSF tests. Such biomarkers would provide a simple and cheap way to identify people with Alzheimer’s pathology before brain damage and symptoms have begun. But developing these tests was so challenging that “at times it seemed almost like a mythical goal”, says Bateman.
To start with, biomarker proteins are 40 times more dilute in the body’s 5 litres of blood than in its 125 millilitres of CSF. And, unlike the CSF, which washes only the brain and the spinal cord, the blood is crowded with proteins from all parts of the body. Moreover, although tau production is mostly confined to the brain, amyloid is produced by cells in many organs, making it harder to interpret amyloid measurements.
Ever since the advent of amyloid-PET scans, dozens of papers claiming to have found a signal for Alzheimer’s disease in blood have been crowding the literature, but these studies were inconsistent and not reproducible, says neurologist Oskar Hansson at Lund University in Sweden. “We needed detection methods orders of magnitude more sensitive than for CSF.”
Such detection methods fall into one of two camps: one uses antibodies that stick to amyloid and tau; the other relies on mass spectrometry, a method used to identify and quantify molecules in a complex mixture. Over the past decade or so, the sensitivities of both approaches have improved to the extent that biomarker tests now consistently deliver accurate results. In the mid-2010s, the first accurate and reliable plasma biomarker test for two amyloid proteins became available, using mass spectrometry. Plasma biomarkers based on tau arrived a few years later.
Drilling down
Researchers continued to look for other variants of Alzheimer’s proteins that could provide ever more accurate biomarkers, in particular certain forms of tau.
One of tau’s main biological functions is to stabilize the inside of neurons, helping to form scaffolds called microtubules. As Alzheimer’s disease progresses, tau proteins become increasingly soluble and fall off the microtubules. They also become stickier, clumping into fibrils. “It is a double whammy of toxicity for neurons,” says neuroscientist Tara Spires-Jones at the University of Edinburgh, UK.
Changes in the chemistry of the tau molecule are what make it more soluble: it becomes studded with phosphate groups, or phosphorylated. The exact position of the phosphate groups on the protein seems to be biologically significant, and forms of tau that are phosphorylated at specific positions have turned out to be useful biomarkers. The tau biomarker that is currently used in CSF diagnostics, p-tau181, is phosphorylated at the 181 position. This variant was the first tau species to be investigated in blood — but a better option was soon found5.
In early 2019, Hansson decided to analyse a variant called p-tau217 in more than 1,400 stored plasma samples from the Swedish BioFINDER-2 cohort, which comprises people with and without dementia. That November, sitting in a conference, he casually opened an e-mail from one of his postdoc researchers. It contained the results of the study. “I was stunned,” he recalls. “The p-tau217 predicted with nearly 100% certainty whether or not the trial participants had Alzheimer’s disease pathology in their brains.” He was next up to speak but his thoughts were reeling and he could no longer concentrate. “It seemed just too good to be true.”
To make sure it was in fact true, together with his colleagues, he analysed samples from an independent cohort in the United States and a cohort in Colombia comprising people with hereditary early-onset Alzheimer’s. The results all lined up and the study was published6 in July 2020. Hansson remembers a time of intense work and no holidays.
The importance of p-tau217 has since been confirmed in many other clinical studies. In fact, it turned out to be so good that some researchers are using it in clinical trials without an accompanying amyloid biomarker. But, although this protein is excellent for diagnostic purposes, it has thrown up a biological mystery.
Studies have shown that it is associated not only with the tau tangles that drive disease symptoms but also with amyloid plaque load5. Scientists had assumed that amyloid precedes tau in the progression of Alzheimer’s, so this unexpected observation has set them rethinking the role of soluble tau in the disease. It suggests that amyloid plaques directly induce a shift in the way that tau is phosphorylated, says Hansson, and that changes in tau phosphorylation begin long before the visible and destructive tau tangles appear in the brain. “This has fuelled the development of therapies aimed at reducing tau production,” he says.
But p-tau217 isn’t the end of the search for biomarkers. For instance, it can’t tell clinicians much about someone’s prognosis. “The development of p-tau217 for Alzheimer’s diagnosis brought us up to the prior gold standard of PET,” says Bateman. “But we need a range of blood biomarkers — to help us to follow the clinical course of the disease, and also to tell us what is happening in the brain when we try to target different aspects of the disease, like inflammation.”
Scientists are particularly interested in the part of the tau molecule that actually anchors it in the tangles. Last year Bateman’s team developed a test for this region and showed that its presence correlated with tangles and with severity of cognitive symptoms7. The researchers are now developing a blood-based assay for it.
Widening the net
Other fluid biomarkers are helping to track further aspects of Alzheimer’s pathology, which could help to define disease stage or provide other clinical insights. “Biomarkers have been very informative in leading us to understand how the brain is changing in this disease,” says Spires-Jones.

Researchers call for a major rethink of how Alzheimer’s treatments are evaluated
One such biomarker is a protein that helps to maintain the structure of glial cells, which themselves provide support to neurons. Over the course of the disease, levels of glial fibrillary acidic protein (GFAP) increase; they decrease when plaques are removed with antibodies. Measuring GFAP can predict future cognitive decline in all types of dementia.
Another protein that can indicate the speed of cognitive decline is neurofilament light chain (NfL), which signals that neurons are breaking down. Its levels predict the intensity of neurodegeneration, although, like GFAP, it doesn’t differentiate Alzheimer’s from other types of dementia8.
Researchers are increasingly turning to popular ‘omics’ technologies to power the search for ever more molecules associated with Alzheimer’s. These technologies comprehensively analyse different types of molecule in an organism, from genes through to proteins. For example, a proteomics study, published in February, identified at least three proteins newly associated with Alzheimer’s9. “The growing list of informative plasma biomarkers enables us to evaluate the neurobiology of Alzheimer’s across the entire spectrum of the disease,” says Aisen.
Speeding up trials
Cheap and quick blood biomarkers have made a big difference in clinical trials for Alzheimer’s. They are already making recruitment into clinical trials easier and faster, without losing accuracy. They allow clinicians to select those who will benefit most, to monitor how well a treatment is controlling the disease and to decide whether and when they need to start another round of therapy.
Trials for two of the three approved drugs for Alzheimer’s are using blood biomarkers to select participants and monitor disease. Both treatments are anti-amyloid antibodies: lecanemab has been approved in the United States, Japan and China, and donanemab in the United States. Trials of an earlier drug that won approval in the United States, aducanumab, took place too early to take advantage of blood markers.
Blood biomarkers will play an important part in new and continuing trials of these and other amyloid-clearing drugs in development, allowing clinicians to recruit people so early that they have no symptoms. This was too difficult and expensive to do without blood tests. Neurologists predict that treating people at this early stage gives the greatest chance of stopping the disease taking hold.
And even before the drugs are in wide use, the blood tests will be a major support to clinicians, who, on the basis of symptom analysis alone, misdiagnose around one-quarter of cases. In a study of more than 1,200 people with cognitive impairment in primary and secondary care in Sweden10, clinicians provided with blood-test results improved the accuracy of their diagnoses to more than 90%.
In the United Kingdom only 65% or so of people with dementia get any diagnosis at all, and just 2% get a CSF measurement or brain scan to allow a molecular diagnosis, says Schott, because memory clinics do not have the capacity to conduct these tests. “Yet a diagnosis is very important for planning, therapy choice and even access to clinical trials.” He is heading a study of 1,100 people in memory clinics around the United Kingdom to see how well blood testing can support clinicians who make diagnoses, and how this might improve outcomes. In collaboration with the London School of Economics and Political Science, the study will also assess the cost-effectiveness of early diagnosis of Alzheimer’s disease.
Fifteen years ago, most pharmaceutical companies scaled back their brain research, or pulled out altogether, seeing no hope. But thanks to the recent breakthroughs, industry investment in Alzheimer’s disease is back in full force. “The genie is out of the bottle now,” says Schott. “We have reached a tipping point — Alzheimer’s disease is a biological process that can be tested for and treated.”
Nature 632, 243-245 (2024)
doi: https://doi.org/10.1038/d41586-024-02535-x
News
NanoMedical Brain/Cloud Interface – Explorations and Implications. A new book from Frank Boehm
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
How lipid nanoparticles carrying vaccines release their cargo
A study from FAU has shown that lipid nanoparticles restructure their membrane significantly after being absorbed into a cell and ending up in an acidic environment. Vaccines and other medicines are often packed in [...]
New book from NanoappsMedical Inc – Molecular Manufacturing: The Future of Nanomedicine
This book explores the revolutionary potential of atomically precise manufacturing technologies to transform global healthcare, as well as practically every other sector across society. This forward-thinking volume examines how envisaged Factory@Home systems might enable the cost-effective [...]
A Virus Designed in the Lab Could Help Defeat Antibiotic Resistance
Scientists can now design bacteria-killing viruses from DNA, opening a faster path to fighting superbugs. Bacteriophages have been used as treatments for bacterial infections for more than a century. Interest in these viruses is rising [...]
Sleep Deprivation Triggers a Strange Brain Cleanup
When you don’t sleep enough, your brain may clean itself at the exact moment you need it to think. Most people recognize the sensation. After a night of inadequate sleep, staying focused becomes harder [...]
Lab-grown corticospinal neurons offer new models for ALS and spinal injuries
Researchers have developed a way to grow a highly specialized subset of brain nerve cells that are involved in motor neuron disease and damaged in spinal injuries. Their study, published today in eLife as the final [...]
Urgent warning over deadly ‘brain swelling’ virus amid fears it could spread globally
Airports across Asia have been put on high alert after India confirmed two cases of the deadly Nipah virus in the state of West Bengal over the past month. Thailand, Nepal and Vietnam are among the [...]
This Vaccine Stops Bird Flu Before It Reaches the Lungs
A new nasal spray vaccine could stop bird flu at the door — blocking infection, reducing spread, and helping head off the next pandemic. Since first appearing in the United States in 2014, H5N1 [...]
These two viruses may become the next public health threats, scientists say
Two emerging pathogens with animal origins—influenza D virus and canine coronavirus—have so far been quietly flying under the radar, but researchers warn conditions are ripe for the viruses to spread more widely among humans. [...]
COVID-19 viral fragments shown to target and kill specific immune cells
COVID-19 viral fragments shown to target and kill specific immune cells in UCLA-led study Clues about extreme cases and omicron’s effects come from a cross-disciplinary international research team New research shows that after the [...]
Smaller Than a Grain of Salt: Engineers Create the World’s Tiniest Wireless Brain Implant
A salt-grain-sized neural implant can record and transmit brain activity wirelessly for extended periods. Researchers at Cornell University, working with collaborators, have created an extremely small neural implant that can sit on a grain of [...]
Scientists Develop a New Way To See Inside the Human Body Using 3D Color Imaging
A newly developed imaging method blends ultrasound and photoacoustics to capture both tissue structure and blood-vessel function in 3D. By blending two powerful imaging methods, researchers from Caltech and USC have developed a new way to [...]
Brain waves could help paralyzed patients move again
People with spinal cord injuries often lose the ability to move their arms or legs. In many cases, the nerves in the limbs remain healthy, and the brain continues to function normally. The loss of [...]
Scientists Discover a New “Cleanup Hub” Inside the Human Brain
A newly identified lymphatic drainage pathway along the middle meningeal artery reveals how the human brain clears waste. How does the brain clear away waste? This task is handled by the brain’s lymphatic drainage [...]
New Drug Slashes Dangerous Blood Fats by Nearly 40% in First Human Trial
Scientists have found a way to fine-tune a central fat-control pathway in the liver, reducing harmful blood triglycerides while preserving beneficial cholesterol functions. When we eat, the body turns surplus calories into molecules called [...]
A Simple Brain Scan May Help Restore Movement After Paralysis
A brain cap and smart algorithms may one day help paralyzed patients turn thought into movement—no surgery required. People with spinal cord injuries often experience partial or complete loss of movement in their arms [...]