The machine-learning algorithm identified a compound that kills Acinetobacter baumannii, a bacterium that lurks in many hospital settings.
Using an artificial intelligence algorithm, researchers at MIT and McMaster University have identified a new antibiotic that can kill a type of bacteria that is responsible for many drug-resistant infections.
If developed for use in patients, the drug could help to combat Acinetobacter baumannii, a species of bacteria that is often found in hospitals and can lead to pneumonia, meningitis, and other serious infections. The microbe is also a leading cause of infections in wounded soldiers in Iraq and Afghanistan.
Acinetobacter baumannii is a species of bacteria commonly found in the environment, such as soil and water, but it can also inhabit human skin and healthcare environments. It's known for its ability to survive on artificial surfaces for extended periods, making hospitals a common site for infection. It primarily affects people with compromised immune systems, causing a range of illnesses including pneumonia, blood infections, and meningitis. A. baumannii is particularly concerning because of its resistance to many antibiotics, leading to its classification as a "superbug." As a result, infections can be challenging to treat, posing a significant threat to healthcare settings.
The researchers identified the new drug from a library of nearly 7,000 potential drug compounds using a machine-learning model that they trained to evaluate whether a chemical compound will inhibit the growth of A. baumannii.
"This finding further supports the premise that AI can significantly accelerate and expand our search for novel antibiotics," says James Collins, the Termeer Professor of Medical Engineering and Science in MIT's Institute for Medical Engineering and Science (IMES) and Department of Biological Engineering. "I'm excited that this work shows that we can use AI to help combat problematic pathogens such as A. baumannii."
Collins and Stokes are the senior authors of the new study, which was published on May 25 in the journal Nature Chemical Biology. The paper's lead authors are McMaster University graduate students Gary Liu and Denise Catacutan and recent McMaster graduate Khushi Rathod.
Drug discovery
Over the past several decades, many pathogenic bacteria have become increasingly resistant to existing antibiotics, while very few new antibiotics have been developed.
Several years ago, Collins, Stokes, and MIT Professor Regina Barzilay (who is also an author on the new study), set out to combat this growing problem by using machine learning, a type of artificial intelligence that can learn to recognize patterns in vast amounts of data. Collins and Barzilay, who co-direct MIT's Abdul Latif Jameel Clinic for Machine Learning in Health, hoped this approach could be used to identify new antibiotics whose chemical structures are different from any existing drugs.
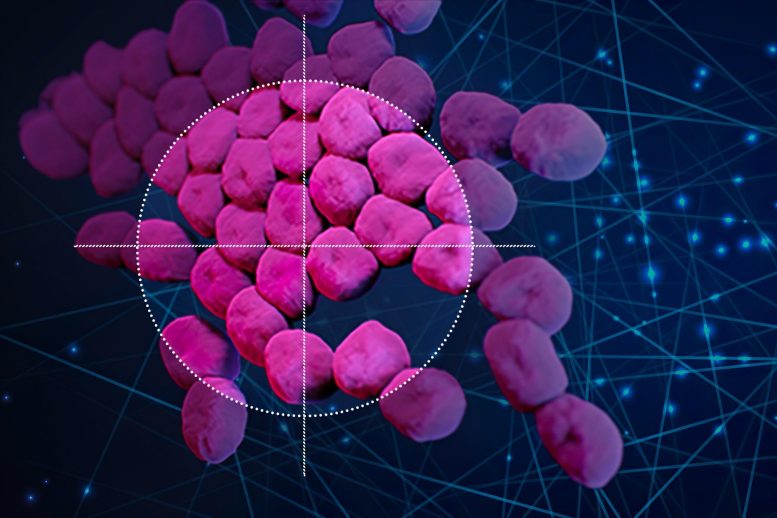
Using an artificial intelligence algorithm, researchers at MIT and McMaster University have identified a new antibiotic that can kill a type of bacteria (Acinetobacter baumannii, pink) that is responsible for many drug-resistant infections. Credit: Christine Daniloff/MIT; Acinetobacter baumannii image courtesy of CDC
In their initial demonstration, the researchers trained a machine-learning algorithm to identify chemical structures that could inhibit growth of E. coli. In a screen of more than 100 million compounds, that algorithm yielded a molecule that the researchers called halicin, after the fictional artificial intelligence system from "2001: A Space Odyssey." This molecule, they showed, could kill not only E. coli but several other bacterial species that are resistant to treatment.
"After that paper, when we showed that these machine-learning approaches can work well for complex antibiotic discovery tasks, we turned our attention to what I perceive to be public enemy No. 1 for multidrug-resistant bacterial infections, which is Acinetobacter," Stokes says.
To obtain training data for their computational model, the researchers first exposed A. baumannii grown in a lab dish to about 7,500 different chemical compounds to see which ones could inhibit growth of the microbe. Then they fed the structure of each molecule into the model. They also told the model whether each structure could inhibit bacterial growth or not. This allowed the algorithm to learn chemical features associated with growth inhibition.
Once the model was trained, the researchers used it to analyze a set of 6,680 compounds it had not seen before, which came from the Drug Repurposing Hub at the Broad Institute. This analysis, which took less than two hours, yielded a few hundred top hits. Of these, the researchers chose 240 to test experimentally in the lab, focusing on compounds with structures that were different from those of existing antibiotics or molecules from the training data.
Those tests yielded nine antibiotics, including one that was very potent. This compound, which was originally explored as a potential diabetes drug, turned out to be extremely effective at killing A. baumannii but had no effect on other species of bacteria including Pseudomonas aeruginosa, Staphylococcus aureus, and carbapenem-resistant Enterobacteriaceae.
This "narrow spectrum" killing ability is a desirable feature for antibiotics because it minimizes the risk of bacteria rapidly spreading resistance against the drug. Another advantage is that the drug would likely spare the beneficial bacteria that live in the human gut and help to suppress opportunistic infections such as Clostridium difficile.
"Antibiotics often have to be administered systemically, and the last thing you want to do is cause significant dysbiosis and open up these already sick patients to secondary infections," Stokes says.
A novel mechanism
In studies in mice, the researchers showed that the drug, which they named abaucin, could treat wound infections caused by A. baumannii. They also showed, in lab tests, that it works against a variety of drug-resistant A. baumannii strains isolated from human patients.
Further experiments revealed that the drug kills cells by interfering with a process known as lipoprotein trafficking, which cells use to transport proteins from the interior of the cell to the cell envelope. Specifically, the drug appears to inhibit LolE, a protein involved in this process.
All Gram-negative bacteria express this enzyme, so the researchers were surprised to find that abaucin is so selective in targeting A. baumannii. They hypothesize that slight differences in how A. baumannii performs this task might account for the drug's selectivity.
"We haven't finalized the experimental data acquisition yet, but we think it's because A. baumannii does lipoprotein trafficking a little bit differently than other Gram-negative species. We believe that's why we're getting this narrow spectrum activity," Stokes says.
Stokes' lab is now working with other researchers at McMaster to optimize the medicinal properties of the compound, in hopes of developing it for eventual use in patients.
The researchers also plan to use their modeling approach to identify potential antibiotics for other types of drug-resistant infections, including those caused by Staphylococcus aureus and Pseudomonas aeruginosa.
News
Plastic Without End: Are We Polluting the Planet for Eternity?
The Kunming Montreal Global Biodiversity Framework calls for the elimination of plastic pollution by 2030. If that goal has been clearly set, why have meaningful measures that create real change still not been implemented? [...]
Scientists Rewire Natural Killer Cells To Attack Cancer Faster and Harder
Researchers tested new CAR designs in NK-92 cells and found the modified cells killed tumor cells more effectively, showing stronger anti-cancer activity. Researchers at the Ribeirão Preto Blood Center and the Center for Cell-Based [...]
New “Cellular” Target Could Transform How We Treat Alzheimer’s Disease
A new study from researchers highlights an unexpected player in Alzheimer’s disease: aging astrocytes. Senescent astrocytes have been identified as a major contributor to Alzheimer’s progression. The cells lose protective functions and fuel inflammation, particularly in [...]
Treating a Common Dental Infection… Effects That Extend Far Beyond the Mouth
Successful root canal treatment may help lower inflammation associated with heart disease and improve blood sugar and cholesterol levels. Treating an infected tooth with a successful root canal procedure may do more than relieve [...]
Microplastics found in prostate tumors in small study
In a new study, researchers found microplastics deep inside prostate cancer tumors, raising more questions about the role the ubiquitous pollutants play in public health. The findings — which come from a small study of 10 [...]
All blue-eyed people have this one thing in common
All Blue-Eyed People Have This One Thing In Common Blue Eyes Aren’t Random—Research Traces Them Back to One Prehistoric Human It sounds like a myth at first — something you’d hear in a folklore [...]
Scientists reveal how exercise protects the brain from Alzheimer’s
Researchers at UC San Francisco have identified a biological process that may explain why exercise sharpens thinking and memory. Their findings suggest that physical activity strengthens the brain's built in defense system, helping protect [...]
NanoMedical Brain/Cloud Interface – Explorations and Implications. A new book from Frank Boehm
New book from Frank Boehm, NanoappsMedical Inc Founder: This book explores the future hypothetical possibility that the cerebral cortex of the human brain might be seamlessly, safely, and securely connected with the Cloud via [...]
Deadly Pancreatic Cancer Found To “Wire Itself” Into the Body’s Nerves
A newly discovered link between pancreatic cancer and neural signaling reveals a promising drug target that slows tumor growth by blocking glutamate uptake. Pancreatic cancer is among the most deadly cancers, and scientists are [...]
This Simple Brain Exercise May Protect Against Dementia for 20 Years
A long-running study following thousands of older adults suggests that a relatively brief period of targeted brain training may have effects that last decades. Starting in the late 1990s, close to 3,000 older adults [...]
Scientists Crack a 50-Year Tissue Mystery With Major Cancer Implications
Researchers have resolved a 50-year-old scientific mystery by identifying the molecular mechanism that allows tissues to regenerate after severe damage. The discovery could help guide future treatments aimed at reducing the risk of cancer [...]
This New Blood Test Can Detect Cancer Before Tumors Appear
A new CRISPR-powered light sensor can detect the faintest whispers of cancer in a single drop of blood. Scientists have created an advanced light-based sensor capable of identifying extremely small amounts of cancer biomarkers [...]
Blindness Breakthrough? This Snail Regrows Eyes in 30 Days
A snail that regrows its eyes may hold the genetic clues to restoring human sight. Human eyes are intricate organs that cannot regrow once damaged. Surprisingly, they share key structural features with the eyes [...]
This Is Why the Same Virus Hits People So Differently
Scientists have mapped how genetics and life experiences leave lasting epigenetic marks on immune cells. The discovery helps explain why people respond so differently to the same infections and could lead to more personalized [...]
Rejuvenating neurons restores learning and memory in mice
EPFL scientists report that briefly switching on three “reprogramming” genes in a small set of memory-trace neurons restored memory in aged mice and in mouse models of Alzheimer’s disease to level of healthy young [...]
New book from Nanoappsmedical Inc. – Global Health Care Equivalency
A new book by Frank Boehm, NanoappsMedical Inc. Founder. This groundbreaking volume explores the vision of a Global Health Care Equivalency (GHCE) system powered by artificial intelligence and quantum computing technologies, operating on secure [...]