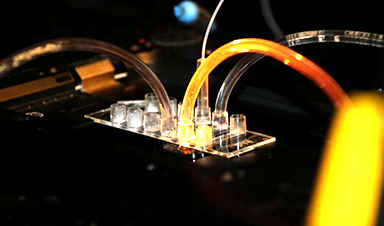
A new tool speeds up development of vaccines and other pharmaceutical products by more than 1 million times while minimizing costs.
More than 40,000 molecules can be synthesized and analyzed within an area smaller than a pinhead. The method, developed through a highly interdisciplinary research effort in Denmark, promises to drastically reduce the amounts of material, energy, and economic cost for pharmaceutical companies.
The method works by using soap-like bubbles as nano-containers. With DNA nanotechnology, multiple ingredients can be mixed within the containers.
“The volumes are so small that the use of material can be compared to using one liter of water and one kilogram of material instead of the entire volumes of water in all oceans to test material corresponding to the entire mass of Mount Everest. This is an unprecedented save in effort, material, manpower, and energy,” says head of the team Nikos Hatzakis, Associate Professor at the Department of Chemistry, University of Copenhagen.
“Saving infinitely [on] amounts of time, energy and manpower would be fundamentally important for any synthesis development and evaluation of pharmaceuticals,” says Ph.D. Student Mette G. Malle, lead author of the article, and currently Postdoc researcher at Harvard University, U.S..
Results within just seven minutes
The work has been carried out in collaboration between the Hatzakis Group, University of Copenhagen, and Associate Professor Stefan Vogel, University of Southern Denmark. The project has been supported by a Villum Foundation Center of Excellence grant. The resulting solution is named “single particle combinatorial lipidic nanocontainer fusion based on DNA mediated fusion”—abbreviated SPARCLD.
The breakthrough involves integration of elements from normally quite distant disciplines: synthetic biochemistry, nanotechnology, DNA synthesis, combinational chemistry, and even Machine Learning, which is an AI (artificial intelligence) discipline.
The method provides results within just seven minutes.
“What we have is very close to a live read-out. This means that one can moderate the setup continuously based on the readings adding significant additional value. We expect this to be a key factor for industry wanting to implement the solution,” says Mette G. Malle.
‘Had to keep things hush-hush’
The individual researchers in the project have several industry collaborations, yet they do not know which companies may want to implement the new high-throughput method.
“We had to keep things hush-hush since we didn’t want to risk for others to publish something similar before us. Thus, we could not engage in conversations with industry or with other researchers that may use the method in various applications,” says Nikos Hatzakis.
Still, he can name some possible applications:
“A safe bet would be that both industry and academic groups involved in synthesis of long molecules such as polymers could be among the first to adopt the method. The same goes for ligands of relevance for pharmaceutical development. A particular beauty of the method [is] that it can be integrated further, allowing for direct addition of a relevant application.”
Here, examples could be RNA strings for the important biotech tool CRISPR, or an alternate for screening and detecting and synthesizing RNA for future pandemic vaccines.
“Our setup allows for integrating SPARCLD with post-combinatorial readout for combinations of protein-ligand reactions such as those relevant for use in CRISPR. Only, we have not been able to address this yet, since we wanted to publish our methodology first.”
News
AI matches doctors in mapping lung tumors for radiation therapy
In radiation therapy, precision can save lives. Oncologists must carefully map the size and location of a tumor before delivering high-dose radiation to destroy cancer cells while sparing healthy tissue. But this process, called [...]
Scientists Finally “See” Key Protein That Controls Inflammation
Researchers used advanced microscopy to uncover important protein structures. For the first time, two important protein structures in the human body are being visualized, thanks in part to cutting-edge technology at the University of [...]
AI tool detects 9 types of dementia from a single brain scan
Mayo Clinic researchers have developed a new artificial intelligence (AI) tool that helps clinicians identify brain activity patterns linked to nine types of dementia, including Alzheimer's disease, using a single, widely available scan—a transformative [...]
Is plastic packaging putting more than just food on your plate?
New research reveals that common food packaging and utensils can shed microscopic plastics into our food, prompting urgent calls for stricter testing and updated regulations to protect public health. Beyond microplastics: The analysis intentionally [...]
Aging Spreads Through the Bloodstream
Summary: New research reveals that aging isn’t just a local cellular process—it can spread throughout the body via the bloodstream. A redox-sensitive protein called ReHMGB1, secreted by senescent cells, was found to trigger aging features [...]
AI and nanomedicine find rare biomarkers for prostrate cancer and atherosclerosis
Imagine a stadium packed with 75,000 fans, all wearing green and white jerseys—except one person in a solid green shirt. Finding that person would be tough. That's how hard it is for scientists to [...]
Are Pesticides Breeding the Next Pandemic? Experts Warn of Fungal Superbugs
Fungicides used in agriculture have been linked to an increase in resistance to antifungal drugs in both humans and animals. Fungal infections are on the rise, and two UC Davis infectious disease experts, Dr. George Thompson [...]
Scientists Crack the 500-Million-Year-Old Code That Controls Your Immune System
A collaborative team from Penn Medicine and Penn Engineering has uncovered the mathematical principles behind a 500-million-year-old protein network that determines whether foreign materials are recognized as friend or foe. How does your body [...]
Team discovers how tiny parts of cells stay organized, new insights for blocking cancer growth
A team of international researchers led by scientists at City of Hope provides the most thorough account yet of an elusive target for cancer treatment. Published in Science Advances, the study suggests a complex signaling [...]
Nanomaterials in Ophthalmology: A Review
Eye diseases are becoming more common. In 2020, over 250 million people had mild vision problems, and 295 million experienced moderate to severe ocular conditions. In response, researchers are turning to nanotechnology and nanomaterials—tools that are transforming [...]
Natural Plant Extract Removes up to 90% of Microplastics From Water
Researchers found that natural polymers derived from okra and fenugreek are highly effective at removing microplastics from water. The same sticky substances that make okra slimy and give fenugreek its gel-like texture could help [...]
Instant coffee may damage your eyes, genetic study finds
A new genetic study shows that just one extra cup of instant coffee a day could significantly increase your risk of developing dry AMD, shedding fresh light on how our daily beverage choices may [...]
Nanoneedle patch offers painless alternative to traditional cancer biopsies
A patch containing tens of millions of microscopic nanoneedles could soon replace traditional biopsies, scientists have found. The patch offers a painless and less invasive alternative for millions of patients worldwide who undergo biopsies [...]
Small antibodies provide broad protection against SARS coronaviruses
Scientists have discovered a unique class of small antibodies that are strongly protective against a wide range of SARS coronaviruses, including SARS-CoV-1 and numerous early and recent SARS-CoV-2 variants. The unique antibodies target an [...]
Controlling This One Molecule Could Halt Alzheimer’s in Its Tracks
New research identifies the immune molecule STING as a driver of brain damage in Alzheimer’s. A new approach to Alzheimer’s disease has led to an exciting discovery that could help stop the devastating cognitive decline [...]
Cyborg tadpoles are helping us learn how brain development starts
How does our brain, which is capable of generating complex thoughts, actions and even self-reflection, grow out of essentially nothing? An experiment in tadpoles, in which an electronic implant was incorporated into a precursor [...]