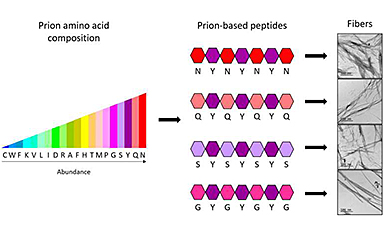
Researchers of the Institute of Biotechnology and Biomedicine (IBB-UAB) have generated four peptides, molecules smaller than proteins, capable of self-assembling in a controlled manner to form nanomaterials. The research, published in the journal ACS Nano, was conducted by Salvador Ventura, Marta Díaz Caballero and Susanna Navarro (IBB-UAB), and included the collaboration of Isabel Fuentes and Francesc Teixidor (Institute of Materials Science of Barcelona, ICMAB-CSIC).
In biotechnology, generating functional synthetic amyloid structures to form nanostructures by imitating the natural generation process is not new. The assembly of proteins into stable fibres allows creating supramolecular shapes that no isolated protein can create, and which are used as nanoconductors, photovoltaic structures, biosensors and catalysts.
Quite recently, researchers began synthesizing prion protein sequences to form nanomaterials. The interest in these sequences lies in the fact that the proteins assemble in a slower and more controlled manner, forming highly ordered, nontoxic nanostructures. However, the fact that the sequence is so long, with over 150 amino acids, makes it very difficult and expensive to synthesise.
“We have demonstrated that an adequate design can permit the size of synthetic prion sequences to be reduced down to only 7 amino acids, while conserving the same properties. The four peptides we have fabricated are the shortest structures of this type created until now, and are capable of forming stable fibril assemblies,” says Salvador Ventura, researcher at the IBB and the UAB Department of Biochemistry and Molecular Biology.
Image Credit: Credit: IBB-UAB
News This Week
Signs of Multiple Sclerosis Show Up in Blood Years Before Symptoms Appear
UCSF scientists clear a potential path toward earlier treatment for a disease that affects nearly 1,000,000 people in the United States. By Levi Gadye In a discovery that could hasten treatment for patients with multiple [...]
Advanced RNA Sequencing Reveals the Drivers of New COVID Variants
A study reveals that a new sequencing technique, tARC-seq, can accurately track mutations in SARS-CoV-2, providing insights into the rapid evolution and variant development of the virus. The SARS-CoV-2 virus that causes COVID has the unsettling [...]
No More Endless Boosters? Scientists Develop One-for-All Virus Vaccine
End of the line for endless boosters? Researchers at UC Riverside have developed a new vaccine approach using RNA that is effective against any strain of a virus and can be used safely even by babies or the immunocompromised. Every [...]
How Are Hydrogels Shaping the Future of Biomedicine?
Hydrogels have gained widespread recognition and utilization in biomedical engineering, with their applications dating back to the 1960s when they were first used in contact lens production. Hydrogels are distinguished from other biomaterials in [...]
Nanovials method for immune cell screening uncovers receptors that target prostate cancer
A recent UCLA study demonstrates a new process for screening T cells, part of the body's natural defenses, for characteristics vital to the success of cell-based treatments. The method filters T cells based on [...]
New Research Reveals That Your Sense of Smell May Be Smarter Than You Think
A new study published in the Journal of Neuroscience indicates that the sense of smell is significantly influenced by cues from other senses, whereas the senses of sight and hearing are much less affected. A popular [...]
Deadly bacteria show thirst for human blood: the phenomenon of bacterial vampirism
Some of the world's deadliest bacteria seek out and feed on human blood, a newly-discovered phenomenon researchers are calling "bacterial vampirism." A team led by Washington State University researchers has found the bacteria are [...]
Organ Architects: The Remarkable Cells Shaping Our Development
Finding your way through the winding streets of certain cities can be a real challenge without a map. To orient ourselves, we rely on a variety of information, including digital maps on our phones, [...]





Leave A Comment