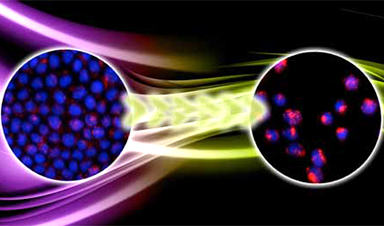
| Diseased cells such as metastatic cancer cells have markedly different mechanical properties that can be used to improve targeted drug uptake, according to a team of researchers at Penn State. | |
| Many labs around the world are developing nanoparticle-based drug delivery systems to selectively target tumors. They rely on a key-and-lock system in which protein keys on the surface of the nanoparticle click into the locks of a highly expressed protein on the surface of the cancer cell. The cell membrane then wraps around the nanoparticle and ingests it. If enough of the nanoparticles and their drug cargo is ingested, the cancer cell will die. | |
| The adhesive force of the lock and key is what drives the nanoparticle into the cell, says Sulin Zhang, professor of engineering science and mechanics. “It is almost universal that whenever there is a driving force for a process, there always is a resistive force. Here, the driving force is biochemical – the protein-protein interaction.” | |
| The resistive force is mechanical, the energy cost of the membrane wrapping around the nanoparticle. Until now, bioengineers only considered the driving force and designed nanoparticles to optimize the chemical interactions, a targeting strategy called “chemotargeting.” Zhang believes they should also take into account the mechanics of the cells to design nanoparticles to achieve enhanced targeting, which forms a new targeting strategy called “mechanotargeting.” |
Image Credit: Zhang lab/vecteezy.com
News This Week
New Research Reveals That Your Sense of Smell May Be Smarter Than You Think
A new study published in the Journal of Neuroscience indicates that the sense of smell is significantly influenced by cues from other senses, whereas the senses of sight and hearing are much less affected. A popular [...]
Deadly bacteria show thirst for human blood: the phenomenon of bacterial vampirism
Some of the world's deadliest bacteria seek out and feed on human blood, a newly-discovered phenomenon researchers are calling "bacterial vampirism." A team led by Washington State University researchers has found the bacteria are [...]
Organ Architects: The Remarkable Cells Shaping Our Development
Finding your way through the winding streets of certain cities can be a real challenge without a map. To orient ourselves, we rely on a variety of information, including digital maps on our phones, [...]
Novel hydrogel removes microplastics from water
Microplastics pose a great threat to human health. These tiny plastic debris can enter our bodies through the water we drink and increase the risk of illnesses. They are also an environmental hazard; found [...]
Researchers Discover New Origin of Deep Brain Waves
Understanding hippocampal activity could improve sleep and cognition therapies. Researchers from the University of California, Irvine’s biomedical engineering department have discovered a new origin for two essential brain waves—slow waves and sleep spindles—that are critical for [...]
The Lifelong Cost of Surviving COVID: Scientists Uncover Long-Term Effects
Many of the individuals released to long-term acute care facilities suffered from conditions that lasted for over a year. Researchers at UC San Francisco studied COVID-19 patients in the United States who survived some of the longest and [...]
Previously Unknown Rogue Immune Key to Chronic Viral Infections Discovered
Scientists discovered a previously unidentified rogue immune cell linked to poor antibody responses in chronic viral infections. Australian researchers have discovered a previously unknown rogue immune cell that can cause poor antibody responses in [...]
Nature’s Betrayal: Unmasking Lead Lurking in Herbal Medicine
A case of lead poisoning due to Ayurvedic medicine use demonstrates the importance of patient history in diagnosis and the need for public health collaboration to prevent similar risks. An article in CMAJ (Canadian Medical Association [...]









Leave A Comment